Table of Contents
Overview
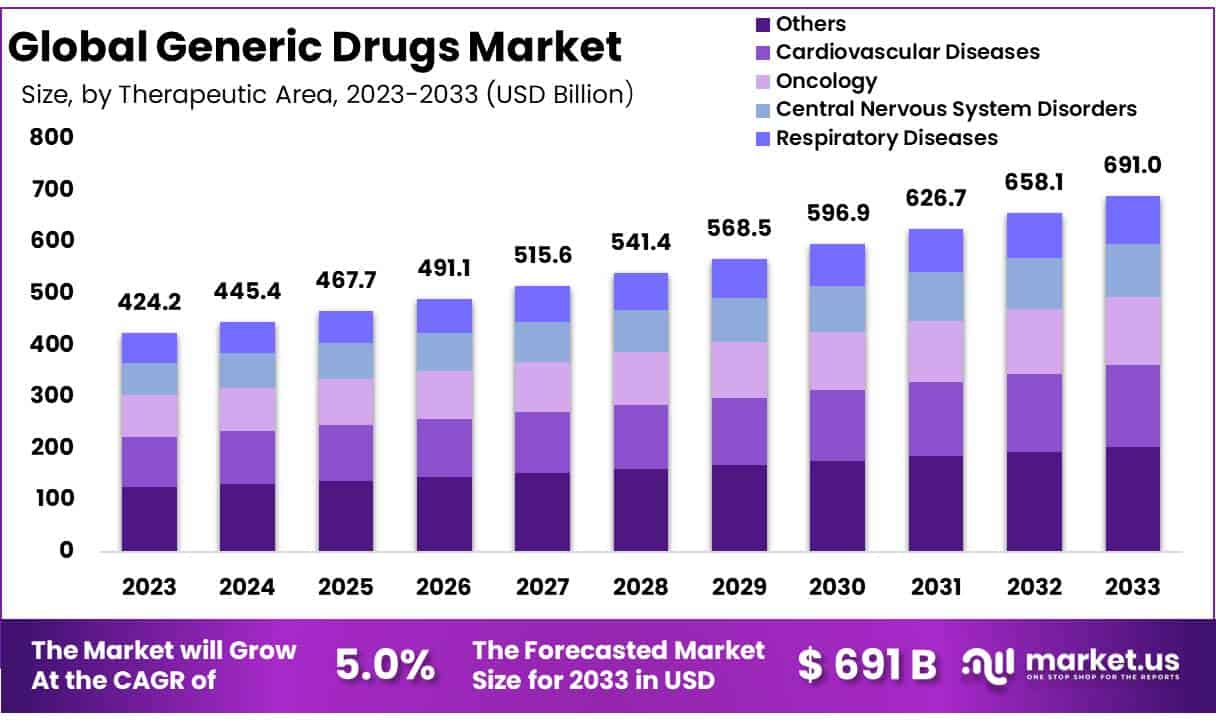
New York, NY – Oct 16, 2025 – The Global Generic Drugs Market size is expected to be worth around USD 691 Billion by 2033 from USD 445.4 Billion in 2024, growing at a CAGR of 5.0% during the forecast period from 2025 to 2033.
The global generic drugs market is experiencing sustained growth, driven by the increasing need for affordable healthcare solutions and the patent expiration of several branded pharmaceuticals. Generic drugs, which are therapeutically equivalent to their branded counterparts, play a vital role in reducing healthcare expenditure while maintaining treatment efficacy and safety.
The market growth is primarily attributed to rising chronic disease prevalence, government initiatives promoting generic substitution, and expanding access to medicines in emerging economies. Additionally, increasing healthcare reforms and favorable regulatory frameworks have accelerated the approval of generic formulations across major markets, including North America, Europe, and Asia-Pacific.
Pharmaceutical companies are focusing on product portfolio expansion, strategic collaborations, and research in complex generics to enhance market competitiveness. The introduction of biosimilars and high-barrier generic drugs is further expected to strengthen the industry outlook.
Despite growth prospects, the market faces challenges such as price erosion, stringent regulatory requirements, and high competition among key players. However, continuous innovation, cost-efficient manufacturing, and supportive healthcare policies are anticipated to sustain market expansion. Overall, the generic drugs industry is projected to witness robust growth over the forecast period, supported by increasing global emphasis on affordable and accessible healthcare solutions.
Key Takeaways
- Global Generic Drugs Market size is expected to be worth around USD 691 Billion by 2033 from USD 445.4 Billion in 2024, growing at a CAGR of 5.0% during the forecast period from 2025 to 2033.
- Based on therapeutic area, the market is segmented into cardiovascular diseases, oncology, central nervous system disorders, respiratory diseases, and others. The “others” segment dominated the market in 2023, accounting for 29.5% of the total share.
- By route of administration, the market includes oral, topical, parenteral, and other forms. The oral segment emerged as the leading category, contributing 66.1% of total revenue in 2023.
- In terms of distribution channel, the market is classified into retail pharmacies, hospital pharmacies, and online pharmacies. The retail pharmacy segment led the market, generating 56.4% of overall revenue during 2023.
- From a regional perspective, North America continued to dominate the global landscape, capturing a 38.4% market share in 2023, supported by strong generic drug adoption rates and favorable healthcare policies.
Regional Analysis
North America remained the leading contributor to the global generic drugs market in 2023, accounting for 38.4% of total revenue. The region is projected to maintain its dominance throughout the forecast period. This strong market position is primarily attributed to the rising prevalence of chronic diseases and the increasing number of patent expirations that encourage the entry of cost-effective generic alternatives.
The United States continues to represent a highly lucrative market, driven by robust demand for affordable medicines. According to the Office of Generic Drugs Annual Report published by the U.S. Food and Drug Administration (FDA), generic drugs account for over 91% of all prescriptions filled in the country. These factors collectively reinforce North America’s leadership in the global generic drugs landscape.
In contrast, the Asia-Pacific region is anticipated to register the highest compound annual growth rate (CAGR) over the forecast period. The region’s growth is supported by the rising incidence of chronic illnesses, rapidly aging populations, and expanding healthcare infrastructure. Moreover, strong pharmaceutical manufacturing capabilities in emerging economies such as China and India further enhance regional market prospects.
According to the Press Information Bureau, India has emerged as the largest global supplier of generic drugs, producing nearly 20% of the world’s generic medicines. These factors collectively indicate a flourishing outlook for the Asia-Pacific generic drugs market in the coming years.
Emerging Trends
- Increased Competition Leading to Lower Prices: The U.S. Food and Drug Administration (FDA) has observed that as more generic manufacturers enter the market, drug prices tend to decrease significantly. For instance, when three generic competitors are present, prices can drop by approximately 20%, and with more competitors, the reduction can reach up to 70–80% compared to the original brand price.
- Government Initiatives to Enhance Access: The U.S. Centers for Medicare & Medicaid Services (CMS) released a preliminary list of 101 generic drugs that would be available for no more than $2 for a month’s supply to those enrolled in the government’s Medicare program. This initiative aims to improve medication adherence by making essential drugs more affordable.
- India’s Expanding Role in Generic Drug Supply: India continues to be a major supplier of generic drugs worldwide, accounting for a significant share of global exports. Indian pharmaceutical companies are preparing to introduce generic versions of popular weight-loss drugs, which could lead to more affordable options for consumers.
Use Cases
- Chronic Disease Management: Generic drugs play a crucial role in managing chronic conditions such as diabetes, hypertension, and high cholesterol. For example, generic statins are widely used to control cholesterol levels, offering a cost-effective alternative to brand-name drugs.
- Cost Savings for Healthcare Systems: The use of generic drugs leads to substantial savings for healthcare systems. In the U.S., generics account for 90% of prescriptions but only 17.5% of total drug spending, resulting in significant cost reductions for patients and insurers.
- Improved Access in Low-Income Countries: Generic drugs have enhanced access to essential medications in low- and middle-income countries. The introduction of generic antiretroviral drugs has significantly reduced the cost of HIV treatment, making it more accessible to patients in need.
- Emergency and Critical Care: In hospital settings, generic injectable drugs are vital for emergency and critical care treatments. Their availability ensures that patients receive timely and effective interventions without the high costs associated with brand-name medications.
- Support for Public Health Programs: Government programs, such as India’s Pradhan Mantri Bharatiya Janaushadhi Pariyojana, utilize generic drugs to provide affordable healthcare solutions. These initiatives have expanded access to essential medicines across various regions.
Frequently Asked Questions on Generic Drugs
- What are generic drugs?
Generic drugs are pharmaceutical products that contain the same active ingredients, strength, dosage form, and route of administration as branded drugs. They offer equivalent therapeutic effects and quality at a significantly lower cost, enhancing medication affordability and accessibility. - How are generic drugs different from branded drugs?
While branded drugs are developed and marketed under patent protection, generic drugs are launched after patent expiration. They provide identical clinical outcomes but are sold at lower prices due to the absence of research and marketing expenses. - Why are generic drugs cheaper?
Generic drugs are less expensive because manufacturers do not bear the initial costs of drug discovery, clinical trials, and marketing. They only need to demonstrate bioequivalence, allowing lower pricing and broader availability across healthcare systems. - How does the approval process for generic drugs work?
Regulatory bodies such as the U.S. FDA evaluate generic drugs for bioequivalence, safety, and quality. The process ensures the drug performs similarly to its branded counterpart, meeting strict pharmaceutical standards before market approval and distribution. - Which therapeutic areas dominate the generic drugs market?
Generic drugs are widely used in cardiovascular, oncology, central nervous system, and respiratory treatments. In 2023, the “others” category, including anti-infectives and gastrointestinal drugs, held the largest market share at approximately 29.5%. - Which route of administration is most common for generic drugs?
The oral route remains the most preferred and widely used method for generic drug administration. In 2023, oral formulations accounted for 66.1% of total market revenue due to ease of use and patient compliance. - What distribution channels are used for generic drugs?
Generic drugs are distributed through retail pharmacies, hospital pharmacies, and online platforms. Retail pharmacies led the market in 2023, generating 56.4% of revenue, supported by strong consumer accessibility and established sales networks. - Which region dominates the global generic drugs market?
North America dominated the global market in 2023, capturing 38.4% of total revenue. High prescription rates, patent expirations, and growing demand for affordable healthcare contribute to the region’s sustained leadership in the industry.
Conclusion
The global generic drugs market is poised for strong and sustained growth, driven by the rising demand for affordable healthcare solutions, patent expirations, and supportive government initiatives. Increasing chronic disease prevalence and healthcare reforms are enhancing generic drug adoption across major regions.
North America continues to lead the market, while Asia-Pacific exhibits the fastest growth, supported by expanding pharmaceutical manufacturing capacities. Despite challenges such as price erosion and regulatory complexities, strategic collaborations, innovation in biosimilars, and favorable policy frameworks are expected to strengthen market expansion.
With its critical role in cost containment and improved healthcare access, the generic drugs market is projected to remain a key component of the global pharmaceutical landscape, reaching approximately USD 691 billion by 2033.


