Table of Contents
Overview
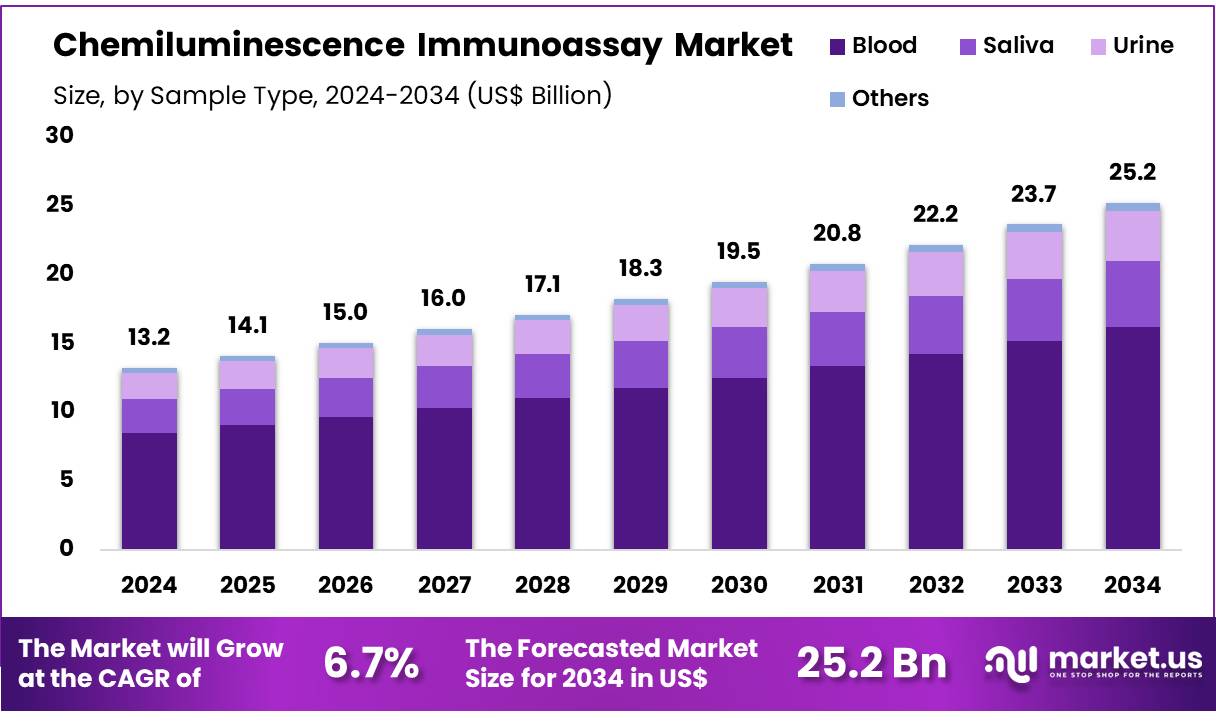
New York, NY – Nov 03, 2025 – The Global Chemiluminescence Immunoassay Market size is expected to be worth around US$ 25.2 Billion by 2034 from US$ 13.2 Billion in 2024, growing at a CAGR of 6.7% during the forecast period 2025 to 2034. North America held a dominant market position, capturing more than a 41.2% share and holds US$ 5.4 Billion market value for the year.
The chemiluminescence immunoassay market has been witnessing significant growth. The expansion of the market has been attributed to the rising global burden of chronic and infectious diseases, coupled with increasing investments in advanced diagnostic technologies. The technique, which utilizes chemiluminescent signals for antigen-antibody detection, has been recognized for its high sensitivity, accuracy, and rapid processing capability.
Increasing demand for early and precise disease diagnosis has been fostering greater adoption across hospitals, clinical laboratories, and research institutions. Automation in diagnostic workflows and the integration of high-throughput analyzers have strengthened laboratory efficiency, which has been contributing to favorable market dynamics. Furthermore, technological advancements such as improved assay sensitivity, enhanced reagent stability, and system miniaturization have supported product innovation across the industry.
North America and Europe have been demonstrating strong penetration due to established healthcare infrastructure and rising screening rates. Meanwhile, Asia-Pacific has been projected to represent substantial future growth, driven by expanding healthcare investments, rapid urbanization, and increasing awareness regarding preventive diagnostics.
The market outlook remains positive, with continued emphasis on precision healthcare, digital laboratory automation, and infectious disease surveillance. Strategic partnerships, research collaborations, and product portfolio expansion are expected to remain key focus areas for major industry participants.
Key Takeaways
- The global Chemiluminescence Immunoassay market generated revenue of US$ 13.2 billion in 2024 and is projected to reach US$ 25.2 billion by 2034, reflecting a CAGR of 6.7%.
- By product type, reagents dominated the market in 2024 with a 61.2% share, compared to analyzers.
- Based on application, therapeutic drug monitoring accounted for the largest share at 45.3%.
- In terms of sample type, blood samples remained the leading segment, representing 64.3% of total revenue.
- Among end users, pharmaceutical and biotechnology companies led the market in 2024, securing 56.2% of the market share.
- North America emerged as the leading regional market, holding 41.2% of the total share in 2024.
Regional Analysis
North America has been identified as the leading region in the Chemiluminescence Immunoassay market, securing a revenue share of 41.2%. This dominance can be attributed to advancements in diagnostic technologies, increased healthcare spending, and rising demand for precise and efficient testing methodologies.
The increasing incidence of chronic diseases, such as cancer, cardiovascular disorders, and autoimmune conditions, has further amplified the adoption of CLIA due to its high sensitivity and specificity in biomarker detection. In November 2023, Medical & Biological Laboratories Co., Ltd., under JSR Life Sciences, introduced the “iStar 500,” an automated and compact CLIA analyzer designed for emergency departments and small to medium-sized laboratories.
The system supports a wide range of tests, including cardiac, liver, reproductive health, tumor markers, and autoimmune diagnostics, demonstrating the region’s commitment to innovation in medical testing. The expanding implementation of technologically advanced diagnostic systems and the growing preference for rapid and cost-effective testing solutions are expected to support continued market expansion across North America.
The Asia Pacific region is projected to witness the fastest CAGR during the forecast period, supported by rising investments in healthcare infrastructure, increasing awareness regarding diagnostic screening, and the growing prevalence of chronic diseases. Notable growth is anticipated in countries such as China, India, and Japan as healthcare systems undergo modernization and access to advanced laboratory technologies increases.
In December 2022, Sysmex received approval in Japan to manufacture and commercialize the HISCL β-Amyloid 1-42 and 1-40 Assay Kits for measuring amyloid beta levels in blood samples, underscoring the region’s emphasis on innovative diagnostic development. The escalating incidence of Alzheimer’s disease, cancer, and infectious conditions is expected to strengthen demand for CLIA solutions.
Government initiatives to enhance healthcare access and rising investments by healthcare providers in diagnostic equipment are further expected to propel market growth. As healthcare requirements in Asia Pacific evolve, the adoption of accurate, rapid, and reliable diagnostic platforms utilizing CLIA technology is anticipated to increase consistently.
Emerging Trend
- Integration with Digital Microfluidics: The integration of digital microfluidic platforms has been gaining significant traction in chemiluminescence immunoassay workflows. These systems enable automated manipulation of samples and reagents on a single chip, reducing manual intervention and contamination risks. The miniaturized design supports faster turnaround times, enabling some platforms to deliver results in under 30 minutes compared to several hours required by conventional systems.
- Enhanced Sensitivity and Dynamic Range: Recent advancements in peroxidase-mediated CLIA reagents have improved assay precision and detection capabilities. Limits of detection have been reported as low as 0.1 pg/mL for specific biomarkers, with linear dynamic ranges extending across five orders of magnitude. This enhancement facilitates accurate measurement of both trace and high-concentration analytes, broadening the clinical applicability of CLIA technology.
- Point-of-Care Chemiluminescence Systems: Compact point-of-care CLIA devices are increasingly being adopted for near-patient diagnostics. The Accre 8 system performs a complete chemiluminescent assay in a single operational step with minimal calibration needs, delivering results in under 15 minutes. Validation involving 239 samples indicated an area under the ROC curve of 0.98 compared to LC-MS/MS systems, confirming its diagnostic reliability and utility for rapid clinical decision-making.
- Synergy with Molecular Diagnostics: CLIA systems are being increasingly integrated with molecular diagnostic platforms to enhance diagnostic accuracy. During the COVID-19 pandemic, chemiluminescent microparticle immunoassays were used alongside RT-PCR for confirmatory SARS-CoV-2 testing. This combined approach achieved sensitivity and specificity rates of 99% and 98%, respectively, reinforcing its value in strengthening clinical and public health surveillance.
Use Cases
- Infectious Disease Screening (HCV): Chemiluminescence immunoassays remain essential for hepatitis C screening in clinical laboratories and blood banks. The FDA-approved VITROS Anti-HCV assay is widely incorporated in national screening programs. With an estimated 2.4 million individuals living with hepatitis C in the United States, high-throughput CLIA systems play a critical role in timely disease detection and linkage to care.
- Cardiac Marker Quantification (Troponin I/T): High-sensitivity CLIA assays for troponin biomarkers are established in emergency settings for early myocardial infarction diagnosis. These assays detect concentrations as low as 0.006 ng/mL with coefficients of variation below 5% at the diagnostic cutoff. Such accuracy supports rapid triage, enabling clinicians to confirm or exclude acute coronary syndrome within one hour, improving patient management efficiency.
- Point-of-Care Vitamin B12 Measurement: The Accre 8 point-of-care CLIA platform has demonstrated strong performance in on-site vitamin B12 testing, delivering results in less than 15 minutes. Evaluation of 239 samples showed a regression slope of 1.44 versus gold-standard LC-MS/MS and an AUC of 0.98, indicating high concordance. This capability supports immediate clinical intervention, particularly in outpatient and resource-limited care settings.
Frequently Asked Questions on Chemiluminescence Immunoassay
- How does CLIA work?
CLIA functions by binding antibodies to target analytes in a sample, followed by a chemical reaction producing light. The emitted light intensity correlates with analyte concentration, enabling precise quantification for clinical diagnosis and monitoring of health conditions. - What are the key advantages of CLIA?
Key benefits include high sensitivity, strong specificity, rapid turnaround time, and capability for automation. These characteristics allow detection of low-level biomarkers, improving accuracy in disease diagnosis and enhancing laboratory efficiency in clinical testing environments. - What are the main applications of CLIA?
CLIA is widely applied in oncology, cardiology, endocrinology, infectious disease detection, autoimmune disorder diagnosis, and therapeutic drug monitoring. These applications support early disease detection, treatment decision-making, and ongoing health monitoring in healthcare facilities. - Which sample types are commonly used in CLIA?
Blood, serum, plasma, saliva, and urine are commonly used sample types for CLIA testing. Blood remains the most preferred sample due to high biomarker concentration and established clinical workflows supporting precise and reliable diagnostic analysis. - Which region holds the largest market share?
North America leads the global market due to high healthcare spending, advanced diagnostic adoption, and strong presence of established laboratory networks. Supportive reimbursement policies and continuous technological innovations further reinforce regional leadership. - Which segment dominates the market by product type?
Reagents dominate the market, accounting for a significant share due to recurring consumption and essential role in routine testing. Their continuous demand in clinical labs sustains strong revenue contributions compared to analyzer systems. - Which factors are driving market growth?
Market growth is driven by increasing chronic disease burden, growing demand for accurate diagnostic tools, technological innovation, and expanding laboratory automation. Rising healthcare investments and adoption of high-performance immunoassay systems contribute to sustained market expansion.
Conclusion
The chemiluminescence immunoassay market has been experiencing robust expansion, driven by rising incidences of chronic and infectious diseases and strong investments in advanced diagnostic technologies. Demand for early and accurate disease detection has supported widespread adoption across hospitals, laboratories, and research facilities.
Advancements in automation, miniaturization, and assay sensitivity have enhanced system performance and efficiency. North America currently leads the market, while Asia Pacific is poised for rapid growth due to increasing healthcare modernization.
Continued innovation, strategic collaborations, and heightened focus on precision diagnostics are expected to sustain market momentum, solidifying CLIA as a critical tool in global healthcare diagnostics.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



