Table of Contents
Overview
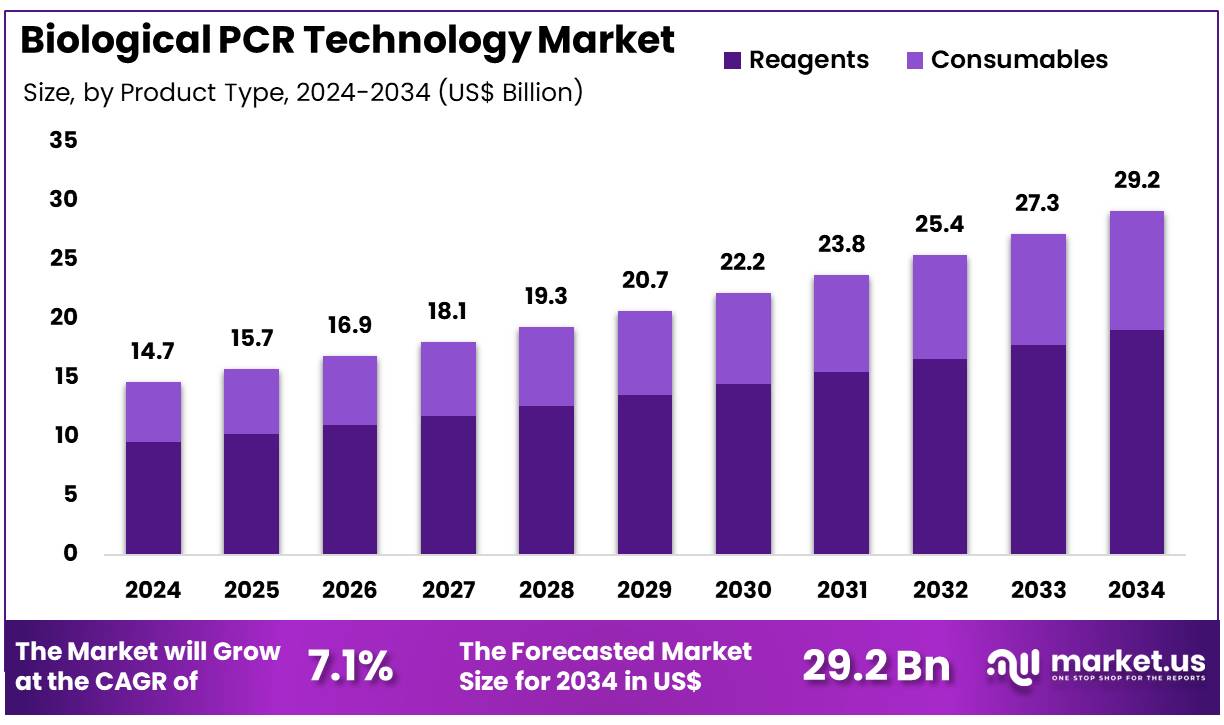
New York, NY – Dec 10, 2025 – Global Biological PCR Technology Market size is expected to be worth around US$ 29.2 Billion by 2034 from US$ 14.7 Billion in 2024, growing at a CAGR of 7.1% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 39.6% share with a revenue of US$ 5.8 Billion.
Biological PCR Technology has been positioned as a core molecular tool that enables precise identification, amplification, and analysis of nucleic acids. Its adoption has been strengthened by rising demand for accurate diagnostics, advanced genetic research, and high-throughput laboratory workflows. The technology operates by replicating targeted DNA or RNA sequences through controlled thermal cycling, which ensures high sensitivity and specificity during detection.
Significant growth has been observed across healthcare, biotechnology, and pharmaceutical sectors, driven by increasing applications in infectious disease testing, oncology research, personalized medicine, and environmental screening. The expansion of research activities worldwide has supported the integration of real-time PCR, digital PCR, and multiplex PCR systems, which offer enhanced quantification capabilities and improved analytical performance.
The market has benefited from technological advancements such as automated platforms, rapid thermal systems, and portable PCR devices that enable point-of-care testing. The demand for reliable diagnostic outcomes has resulted in the deployment of PCR solutions across clinical laboratories, research institutes, and industrial testing facilities. In addition, investments in genomic projects and biomarker development have accelerated the use of PCR workflows for precision-driven scientific outcomes.
The growth of Biological PCR Technology can be attributed to its broad applicability, cost-efficient operation, and ability to deliver accurate results within reduced turnaround times. The continued emphasis on molecular diagnostics and global health preparedness is expected to support sustained adoption, positioning PCR as a foundational technology within modern biological and medical research ecosystems.
Key Takeaways
- In 2024, the Biological PCR Technology market generated US$ 14.7 Billion in revenue, supported by a 7.1% CAGR, and is projected to reach US$ 29.2 Billion by 2034.
- The product type segment consists of reagents and consumables, with reagents accounting for the largest share at 65.3% in 2024.
- Based on application, the market is categorized into diagnostic laboratories and molecular testing laboratories, with diagnostic laboratories holding 72.4% of the market in 2024.
- North America dominated the industry in 2024, securing a 39.6% share of the global market.
Regional Analysis
North America is Leading the Biological PCR Technology Market
North America accounted for the largest share of 39.6% in 2024, supported by sustained demand for rapid and accurate diagnostic solutions, advancements in molecular biology research, and continued public health preparedness programs. Although dedicated PCR-specific funding is embedded within broader scientific allocations, the overall National Institutes of Health budget reached approximately US$47.311 billion in fiscal year 2024, a portion of which was directed toward molecular diagnostic research and development. This consistent governmental commitment has reinforced innovation across PCR platforms.
Industry performance further reflects strong regional adoption. Thermo Fisher Scientific reported annual revenue of US$42.88 billion in 2024, with its Life Sciences Solutions segment significantly engaged in PCR instruments and reagents. Abbott Laboratories recorded US$9.341 billion in sales within its Diagnostics segment in the same year, demonstrating the solid commercial momentum for molecular diagnostic technologies in the region.
Asia Pacific is Expected to Register the Highest CAGR
Asia Pacific is projected to record the fastest growth during the forecast period due to expanding healthcare infrastructure, increasing incidence of infectious and chronic diseases, and rising investment from both government bodies and private stakeholders. Regional initiatives continue to strengthen diagnostic capacity; India’s Department of Health Research implemented a Central Sector Scheme to establish 163 Viral Research & Diagnostic Laboratories between fiscal years 2021–22 and 2025–26, directly boosting PCR testing capabilities.
China continues to advance medical device research through substantial governmental support for domestic molecular diagnostics manufacturers. Commercial expansion also remains strong, with Bio-Rad Laboratories reporting that Asia Pacific contributed 20.3% of its total sales in 2024, driven by growth in its Clinical Diagnostics segment. These developments are expected to accelerate the adoption of molecular technologies across diverse applications.
Emerging Trends
- Digital PCR for Environmental Surveillance
Digital PCR is being increasingly applied in environmental monitoring, supported by its endorsement under the CDC’s National Wastewater Surveillance System. By mid-2022, over 70 % of U.S. states operated laboratories with dPCR platforms, enabling sub-two-hour pathogen detection and strengthening early outbreak alert capabilities. - Multiplexed Real-Time Assays in Newborn Screening
Real-time PCR assays are being expanded through multiplexing to enable simultaneous detection of multiple conditions. Since 2008, SCID screening has been adopted in 25 U.S. public health programs, doubling recognized SCID prevalence and demonstrating capacity to integrate assays such as SMA testing without additional instrumentation. - Integration of Genomic Sequencing and Bioinformatics
The CDC’s Advanced Molecular Detection initiative is facilitating broader integration of genomic sequencing and bioinformatics with PCR-based diagnostics. This combined approach supports accelerated pathogen identification, improved variant tracking, and reduced outbreak response timelines through more rapid detection of genetic changes. - Regulatory Expansion of Nucleic Acid-Based Tests
The FDA’s Center for Devices and Radiological Health has expanded approvals for nucleic acid-based diagnostic tests, including numerous PCR panels. This increasing regulatory activity highlights ongoing advancements in assay design, analytical performance, and validation standards across clinical and public health laboratories.
Use Cases
- Newborn SMA Screening
By early 2024, universal newborn screening for spinal muscular atrophy had been implemented across all U.S. states through PCR-based assays. This nationwide coverage ensures near-complete early detection in newborns, enabling prompt therapeutic intervention and improved long-term health outcomes. - Congenital CMV Surveillance
A universal PCR-based screening initiative for congenital cytomegalovirus in Minnesota during 2023 reported a prevalence of 0.3 % among live births. Nearly all initial positives were confirmed through follow-up testing, ensuring timely linkage of affected infants to appropriate clinical management. - Wastewater-Based Pathogen Monitoring
PCR methodologies are widely utilized in wastewater surveillance to track community pathogen circulation. More than 70 % of U.S. states report weekly dPCR-based wastewater data on over 30 biological targets, generating actionable insights that support public health response planning and resource allocation. - SCID Newborn Screening
Real-time PCR assays for severe combined immunodeficiency are employed by 25 state programs, identifying cases at twice the previously estimated incidence. High-throughput PCR systems in these laboratories contribute to survival rates exceeding 90 % among infants receiving early treatment.
Frequently Asked Questions on Biological PCR Technology
- How does PCR work?
PCR functions through cyclical temperature changes that drive DNA denaturation, primer annealing, and extension. These cycles enable the production of millions of copies of a target sequence, supporting accurate genetic identification and analysis across clinical, biological, and environmental applications. - What are the major types of PCR?
PCR types include conventional PCR, real-time quantitative PCR, multiplex PCR, and digital PCR. These variants are used for diverse analytical needs, offering improved quantification, enhanced sensitivity, and greater throughput in research, clinical diagnostics, and molecular biology workflows. - What are the key applications of PCR?
PCR applications span infectious disease diagnostics, oncology, genetic screening, food safety, forensic testing, and environmental monitoring. Its ability to detect small quantities of DNA enables rapid, reliable analysis that supports clinical decision-making, research innovation, and regulatory compliance. - What advantages does PCR offer?
PCR advantages include high sensitivity, specificity, rapid turnaround, and compatibility with numerous sample types. These strengths support accurate detection of genetic material even at minimal concentrations, enhancing diagnostic reliability and enabling broad adoption across laboratories and healthcare systems. - What drives growth in the Biological PCR Technology market?
Market growth is driven by rising demand for molecular diagnostics, increased prevalence of infectious diseases, and expanding applications in oncology and genomics. Advancements in real-time and digital PCR platforms also enhance adoption across clinical laboratories and research institutions globally. - Which industries use PCR technology most extensively?
Extensive PCR adoption is observed in healthcare diagnostics, pharmaceutical research, biotechnology, forensic laboratories, agricultural testing, and food safety monitoring. These sectors rely on PCR for precise genetic detection, quality assurance, and compliance with regulatory standards. - What regional markets dominate PCR adoption?
North America and Europe dominate PCR adoption due to established healthcare infrastructure, significant R&D activities, and strong regulatory frameworks. Asia-Pacific is experiencing rapid growth as expanding diagnostic capacity and government investments support wider molecular testing deployment. - Who are the major players in the market?
Major companies include Thermo Fisher Scientific, Bio-Rad Laboratories, Roche, Qiagen, and Agilent Technologies. These organizations provide advanced PCR instruments, reagents, and software solutions that support high-performance molecular diagnostics and research activities worldwide.
Conclusion
The global Biological PCR Technology market is expected to experience sustained expansion, supported by rising diagnostic demand, continuous technological improvements, and broader adoption across healthcare, research, and industrial sectors. Increasing integration of digital and real-time PCR platforms, coupled with strong government and private investments, has strengthened analytical capabilities and improved testing efficiency.
Regional growth is led by North America, while Asia Pacific is projected to register the fastest gains due to expanding healthcare infrastructure. The market outlook remains positive, driven by the technology’s versatility, high accuracy, and critical role in modern molecular diagnostics and genomic research.


