Table of Contents
Overview
New York, NY – Oct 31, 2025 – Global Cancer Therapeutics and Biotherapeutics Market size is expected to be worth around US$ 417.2 Billion by 2034 from US$ 184.5 Billion in 2024, growing at a CAGR of 8.5% during the forecast period 2025 to 2034. In 2023, North America led the market, achieving over 41.6% share with a revenue of US$ 76.8 Billion.
A strategic communication has been prepared to announce developments in cancer therapeutics and biotherapeutics. The initiative can be positioned as a transformative advancement in oncology treatment solutions. The primary focus is placed on innovation, patient outcomes, and technological evolution.
A leading role in cancer research and biologically driven therapeutics is being strengthened with the introduction of advanced platforms targeting solid tumors, hematologic malignancies, and rare cancer types. The growth of the cancer therapeutics market can be attributed to rising cancer incidence, continuous technological improvements, and strong investment in immuno-oncology and precision-medicine programs. In addition, the adoption of biotherapeutics such as monoclonal antibodies, cell-based therapies, and immune checkpoint inhibitors is accelerating due to favorable clinical outcomes and increasing regulatory support.
The development pipeline includes next-generation immunotherapies, targeted therapies, and biologically engineered molecules designed to enhance treatment precision and minimize toxicity. Research collaborations with leading academic institutions and biotechnology partners have been initiated to support accelerated discovery and development processes. Clinical advancements are being driven through multi-phase trials that emphasize safety, efficacy, and long-term survival benefits.
Demand for effective and minimally invasive cancer treatments is rising, and a strengthened commitment to innovation is expected to expand access to breakthrough therapies globally. Commercial readiness programs, manufacturing scale-up strategies, and regulatory engagements are underway to ensure timely delivery to healthcare providers and patients.
The initiative reflects a long-term vision to redefine cancer care, improve quality of life for patients worldwide, and support the global transition toward biologically precise cancer therapies.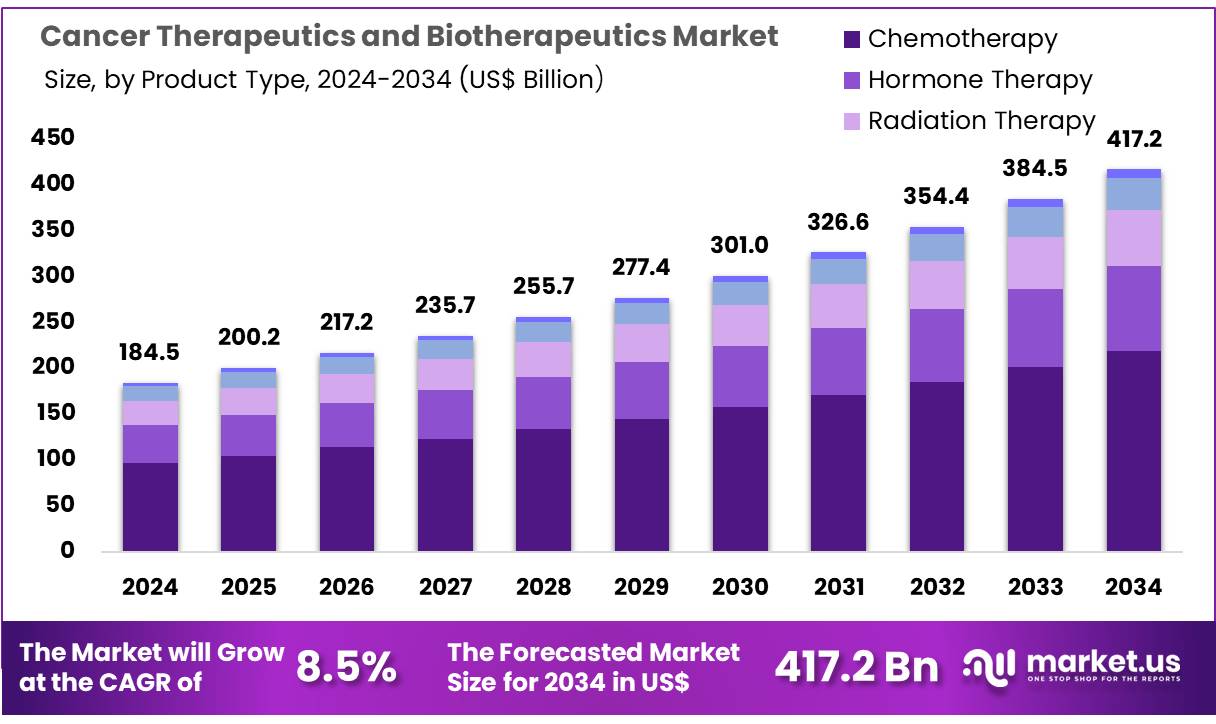
Key Takeaways
- In 2024, the cancer therapeutics and biotherapeutics market generated revenue of US$ 184.5 billion. A CAGR of 8.5% has been recorded, and the market size is projected to reach US$ 417.2 billion by 2024.
- By product type, the market is segmented into chemotherapy, hormone therapy, radiation therapy, biotherapy, and others. Chemotherapy emerged as the leading category in 2024, accounting for 52.4% of the market share.
- Based on application, the market includes lung cancer, blood cancer, breast cancer, prostate cancer, and others. Lung cancer held the largest share in 2024, representing 43.2% of the market.
- In terms of end-use, the market is classified into hospitals, ambulatory surgical centers (ASC), and others. Hospitals dominated this segment, capturing 61.3% of total revenue in 2024.
- Regionally, North America led the global market in 2024, securing 41.6% of the overall share.
Regional Analysis
North America remains the leading region in the Cancer Therapeutics and Biotherapeutics Market
North America accounted for the largest revenue share of 41.6%, driven by continued advancements in immunotherapy, targeted therapies, and significant investments in oncology research. A well-established healthcare system and rising awareness regarding advanced cancer treatment solutions have supported the demand for innovative, personalized therapies.
An important development contributing to regional growth was the U.S. FDA approval of 11 oncology drugs and biologics in 2023 specifically for pediatric cancer patients. This regulatory milestone reflects a strong regional commitment to expanding treatment options for younger patient groups, reinforcing market expansion prospects.
Moreover, the increasing incidence of cancer and growing acceptance of biologic therapies, including monoclonal antibodies and gene therapies, have accelerated market growth in the region. A rising emphasis on precision oncology, coupled with strategic collaborations between pharmaceutical manufacturers and research organizations, is anticipated to sustain strong market performance across North America.
Asia Pacific is expected to record the fastest CAGR during the forecast period
The Asia Pacific region is projected to witness the highest growth rate due to a rising cancer burden, improvement in healthcare infrastructure, and expanding access to advanced treatment modalities. Key countries such as China, India, and Japan are expected to drive regional growth, supported by increasing focus on cancer care, clinical research activities, and government initiatives promoting oncology innovation.
Growing cancer prevalence in these economies, along with heightened demand for novel biotherapeutics, is anticipated to strengthen market expansion. The region’s ongoing healthcare modernization and rapid adoption of advanced treatments, including immunotherapies, targeted therapies, and gene-based interventions, further support this outlook.
Additionally, the emergence of regional and global pharmaceutical players investing in biologic drug development, along with supportive policy frameworks and research funding, is expected to accelerate market penetration. As efforts intensify toward customized and highly effective oncology therapies, the cancer therapeutics and biotherapeutics market in Asia Pacific is likely to experience substantial growth throughout the forecast period.
Use Cases
- Relapsed/Refractory Large B-Cell Lymphoma (CAR T-Cell Therapy)
In the ZUMA-3 study evaluating brexucabtagene autoleucel (Tecartus) for adults with B-cell precursor acute lymphoblastic leukemia, more than half of the over 50 participants achieved complete remission within three months, with many maintaining remission for over one year. - Unresectable or Metastatic Melanoma (TIL Therapy)
Lifileucel tumor-infiltrating lymphocyte therapy demonstrated objective responses in a considerable proportion of heavily pretreated melanoma patients following tumor tissue harvest. This single-dose treatment offers a critical therapeutic option for approximately 5,000 patients globally who are ineligible for checkpoint inhibitor therapies. - CD19-Positive B-Cell Precursor ALL (Bispecific Antibody)
Blinatumomab, a bispecific CD3-CD19 antibody, has been administered to more than 2,000 patients since accelerated approval. It is indicated for adults and children as young as one month with minimal residual disease ≥0.1% or relapsed or refractory B-cell precursor acute lymphoblastic leukemia. - Multiple Myeloma (Allogeneic CAR T Trial)
Early-stage clinical trials of CB-011, an allogeneic CRISPR-engineered anti-BCMA CAR T-cell therapy, are currently underway at over 10 clinical sites. Each cohort aims to enroll 30–40 patients, with primary evaluation endpoints focused on safety and pharmacokinetics. - Synovial Sarcoma (TCR Gene Therapy)
Afamitresgene autoleucel, a TCR-based therapy targeting the MAGE-A4 antigen in HLA-A\02-positive patients, has shown notable outcomes. The pivotal study enrolled approximately 80 participants, achieving objective response rates near 25%, establishing a significant proof of concept for TCR therapies in solid tumors.
Frequently Asked Questions on Cancer Therapeutics and Biotherapeutics
- What are cancer therapeutics and biotherapeutics?
Cancer therapeutics and biotherapeutics refer to medical treatments designed to prevent, control, or eliminate cancer cells. These include chemotherapy, targeted therapy, immunotherapy, hormone therapy, and biologic drugs that harness cellular and molecular mechanisms to treat cancer effectively. - How do biotherapeutics differ from traditional cancer treatments?
Biotherapeutics differ from traditional treatments by using biological agents such as monoclonal antibodies, cytokines, and gene-based therapies. These therapies target cancer cells more precisely, aiming to reduce damage to healthy tissue and improve treatment outcomes compared to conventional chemotherapy or radiation. - What types of cancers are commonly treated with biotherapeutics?
Biotherapeutics are widely used for treating cancers such as lung, breast, blood, and prostate cancer. Their application has expanded due to increasing effectiveness in targeting cancer-specific pathways, improving patient survival, and supporting personalized treatment approaches across various cancer types. - What are the key advantages of biotherapeutic cancer treatments?
Biotherapeutic treatments offer advantages such as targeted mechanisms of action, reduced side effects, and improved effectiveness in certain cancers. They support personalized medicine by addressing tumor-specific biological markers, leading to enhanced therapeutic outcomes and better long-term patient management. - What is driving growth in the cancer therapeutics and biotherapeutics market?
Market growth is driven by rising cancer prevalence, advancements in biologic technologies, and increased investments in oncology research. Expanding adoption of personalized medicine and the introduction of innovative therapies are also contributing significantly to global market expansion. - Which region dominates the global cancer therapeutics market?
North America dominates the global market due to advanced healthcare systems, strong presence of key industry players, and high adoption of innovative cancer treatments. Increasing research funding and strong regulatory approvals further support regional leadership and market expansion. - Which therapy segment holds the largest market share?
Chemotherapy currently holds the largest share, accounting for 52.4% of the market in 2024. Despite growing adoption of targeted and biologic therapies, chemotherapy remains widely used due to established clinical application and broad treatment relevance across cancer types. - What future trends are expected in the market?
Future trends include rapid expansion of immunotherapies, gene therapies, and precision oncology solutions. Increasing collaboration between biotechnology firms and research institutes, along with growing focus on biomarker-driven therapies, is expected to accelerate innovation and market development.
Conclusion
The advancements in cancer therapeutics and biotherapeutics reflect a transformative shift toward precision-driven, biologically engineered treatment modalities. Continuous innovation in immunotherapies, targeted therapies, and cell-based interventions, supported by strong regulatory momentum and industry-academic collaborations, is accelerating clinical progress and improving patient outcomes.
North America currently leads global adoption owing to advanced infrastructure and research investments, while Asia Pacific is poised for rapid expansion driven by rising cancer prevalence and growing access to advanced care.
With expanding clinical pipelines, manufacturing scale-up initiatives, and increasing emphasis on personalized oncology, the global market is positioned to witness sustained growth and enhanced therapeutic accessibility in the coming years.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



