Table of Contents
Overview
New York, NY – July 19, 2025: The Sterility Indicators Market is projected to grow from USD 1.1 billion in 2023 to USD 3.5 billion by 2033. This reflects a strong CAGR of 12.4% during the forecast period from 2024 to 2033. The growth is driven by rising infection control awareness in healthcare and labs. Sterility indicators are essential tools for validating sterilization processes. Their adoption is increasing across hospitals, pharmaceutical firms, and medical device manufacturers to ensure patient safety and compliance with sterilization protocols.
North America held the largest market share in 2023, accounting for 39.4% of global revenue. This dominance is due to strict healthcare regulations and a strong emphasis on infection control. Facilities in the U.S. and Canada are focusing on improving sterilization standards. The region benefits from advanced infrastructure, regulatory support, and rising healthcare investments. With increasing hospital-acquired infection (HAI) cases, the region continues to demand more effective sterility assurance products to meet growing safety and compliance requirements.
The World Health Organization reports that hospital-acquired infections affect 7% of hospitalized patients in developed countries. In developing regions, this figure rises to 10%. These statistics highlight the urgent need for robust sterilization and monitoring systems. Sterility indicators help detect potential lapses in sterilization and ensure reliability. Their use reduces the risks of infections during medical procedures. As awareness of such risks increases, hospitals and labs are adopting advanced monitoring technologies to protect both patients and staff.
According to the CDC, one in 25 hospitalized patients in the U.S. acquires a healthcare-associated infection each year. This places significant emphasis on sterility assurance. Rapid-readout and biological indicators have become popular due to their accuracy and speed. These tools enhance workflow and decision-making in fast-paced environments like surgical centers and pharmaceutical plants. As a result, healthcare providers are shifting toward faster, more efficient sterilization validation methods to meet both patient safety goals and regulatory expectations.
The increasing complexity of sterilization processes creates growth opportunities for the market. Evolving technologies in medical devices and pharmaceutical products demand highly reliable sterilization validation. Regulatory authorities are also strengthening their sterilization guidelines. This pushes manufacturers to adopt comprehensive sterility indicators for quality assurance. The demand for sterility assurance is expanding across new product development, cleanroom environments, and clinical trial setups. This trend ensures steady market expansion as healthcare innovations continue to evolve globally.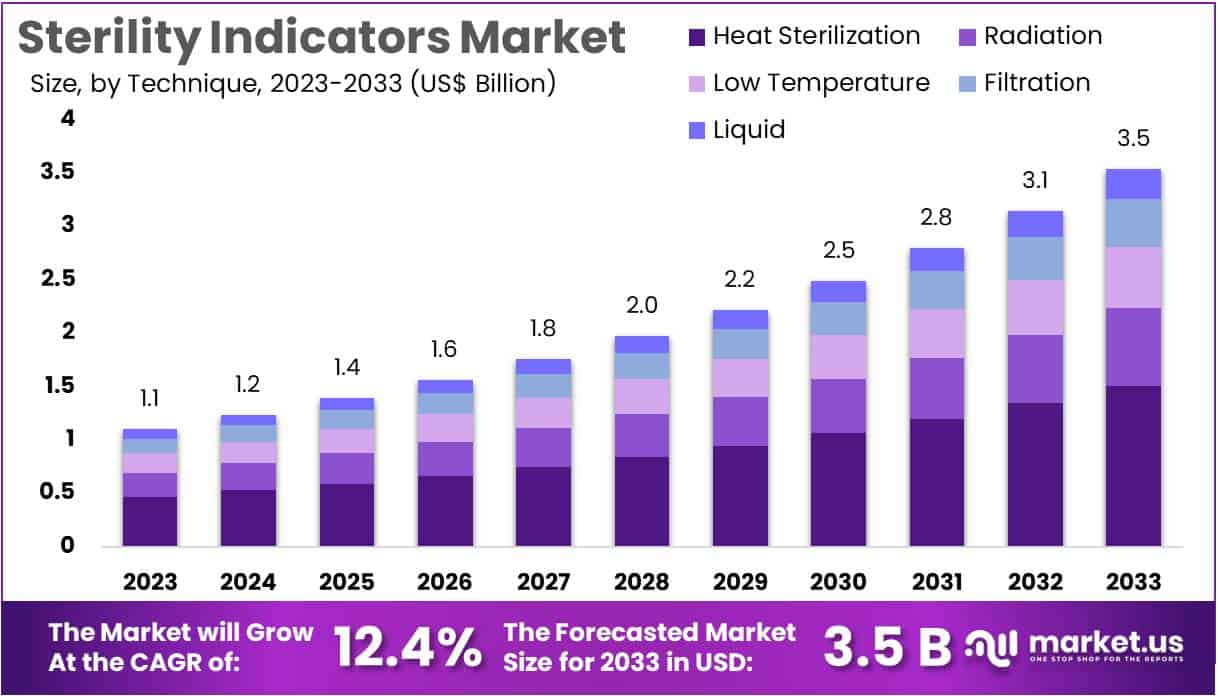
Key Takeaways
- In 2023, the sterility indicators market earned $1.1 billion in revenue and is set to hit $3.5 billion by 2033, growing steadily.
- The biological type led the type segment in 2023, holding a dominant 60.7% market share over its chemical counterparts.
- Heat sterilization emerged as the top technique in 2023, capturing 42.6% of the market due to its broad use and effectiveness.
- Hospitals were the primary end users of sterility indicators, accounting for the largest revenue share of 36.4% across all user segments.
- North America dominated the global market in 2023, commanding a substantial 39.4% share, driven by strong healthcare regulations and infrastructure.
Regional Analysis
North America currently leads the Sterility Indicators Market, accounting for the largest revenue share of 39.4% in 2023. This dominance is driven by heightened awareness of infection control, strict regulatory requirements, and a growing emphasis on patient safety. The widespread adoption of sterility indicators in hospitals stems from the need to prevent healthcare-associated infections (HAIs), with data showing that one in every 25 hospitalized patients in the U.S. acquires an HAI annually. The region’s increasing surgical volumes and advanced medical device usage further fuel demand for reliable sterilization validation. Additionally, substantial investments by U.S. and Canadian healthcare facilities in infection prevention align with regulatory frameworks from the CDC and FDA, reinforcing the importance of stringent sterility assurance protocols.
Meanwhile, the Asia Pacific region is expected to register the highest CAGR during the forecast period due to rapid improvements in healthcare infrastructure and a rising focus on infection control. Countries like India, China, and Indonesia are witnessing increased healthcare access and urbanization, leading to greater exposure to infectious diseases. According to the WHO, South-East Asia accounts for 43% of global tuberculosis cases, highlighting the urgency of robust sterilization standards. Government-led modernization efforts, expanding pharmaceutical and biotech industries, and regional collaborations with international manufacturers are further accelerating the adoption of sterility indicators in the region’s clinical and laboratory settings.
Segmentation Analysis
In 2023, the biological segment dominated the sterility indicators market with a 60.7% share, driven by its high accuracy in detecting microbial contamination. These indicators use highly resistant bacterial spores, offering a more realistic and dependable verification of sterilization processes. Their ability to mimic actual sterilization conditions makes them a preferred choice in hospitals, pharmaceutical manufacturing, and other critical environments. Rising regulatory pressures across healthcare and life sciences sectors have further fueled adoption, especially as guidelines increasingly prioritize high-assurance sterilization validation methods.
Heat sterilization emerged as the leading technique, holding a significant 42.6% market share in 2023. Known for its effectiveness against a wide range of microorganisms, including resilient bacterial spores, heat sterilization remains a cost-effective and reliable method. Its widespread use in hospitals and laboratories, where large-scale sterilization cycles are common, continues to support its dominance. Additionally, technological improvements in sterilization equipment have enhanced process efficiency, making heat-based methods even more attractive for high-throughput settings that demand consistent sterilization standards.
Hospitals led the end-user segment with a 36.4% revenue share, primarily due to the volume of sterilization procedures required to maintain infection control and ensure patient safety. As surgical procedures rise—particularly in emerging economies—there is growing reliance on sterility indicators to comply with stringent protocols. Enhanced investments in infection prevention programs, combined with greater awareness among medical staff, continue to drive demand. With the global focus on reducing healthcare-associated infections, hospitals are expected to maintain a strong demand for advanced sterilization verification tools.
By Type
- Chemical
- Class 1
- Class 2
- Class 3
- Class 4
- Class 5
- Biological
- Spore Suspensions
- Self-Contained Vials
- Spore Ampoules
- Spore Strips
By Technique
- Heat Sterilization
- Radiation
- Low Temperature
- Filtration
- Liquid
By End-user
- Hospitals
- Clinical Laboratories/Research Centers
- Pharmaceutical Companies
- Medical Device Companies
- Others
Key Players Analysis
Leading players in the sterility indicators market are heavily focused on innovation and strategic growth initiatives to strengthen their market position. By expanding their product portfolios, these companies aim to meet the complex and evolving demands of the healthcare and pharmaceutical sectors. A strong emphasis on research and development allows them to create more sensitive, accurate, and efficient indicators, enhancing sterility assurance across various applications. This ongoing innovation is key to meeting stringent regulatory standards and supporting global infection control efforts.
In addition to product development, market leaders are pursuing strategic acquisitions, partnerships, and geographic expansion to broaden their global reach. These collaborations help leverage existing networks and expertise, enabling faster entry into emerging markets. Companies are also investing in marketing and education campaigns to build brand recognition and promote the importance of sterility indicators among end-users. With growing demand for quality assurance in healthcare, these strategies are expected to drive long-term market growth and customer loyalty.
Emerging Trends
Shift to Biological Indicators for Higher Accuracy
Many hospitals and labs are now choosing biological indicators over chemical ones. This shift is because biological indicators give more accurate results. They contain real bacterial spores, which respond to sterilization like actual microorganisms. This makes them better at confirming whether sterilization really works. Chemical indicators change color, but they may not detect deeper problems. Biological indicators mimic real-world sterilization conditions. This means they offer more trust in safety, especially during surgeries. With infection control becoming more important, this trend is growing fast. Healthcare and pharmaceutical sectors prefer using these indicators to reduce risks and meet high safety standards.
Faster Result Times Through Innovation
Earlier, biological sterility indicators needed 24 to 48 hours to give results. That caused delays in hospitals and labs. But now, new technologies are changing this. Modern indicators can deliver results in just a few hours. Some can even work within one hour. This saves time and keeps work moving faster. Hospitals can reuse surgical tools more quickly. Pharmaceutical companies can also check equipment safety sooner. These fast-read indicators help reduce downtime and improve efficiency. The demand for quicker results is rising, especially in emergency care and production labs. Faster response times mean better productivity and patient safety.
Integration with Digital Monitoring Systems
Sterility indicators are now being used with digital tracking tools. These tools automatically record sterilization data. This reduces manual errors and improves accuracy. Hospitals and labs no longer need to write down each sterilization cycle. Instead, systems track and store data in real-time. This also makes it easier to meet legal and safety guidelines. Audits and inspections become smoother with digital records. Many facilities use barcode scanning and smart sensors with their indicators. These smart systems help manage sterilization loads more efficiently. Digital integration is becoming a must-have feature for modern healthcare and pharmaceutical operations.
Customized Indicators for Complex Devices
Not all medical devices are easy to sterilize. Some tools, like endoscopes and robotic arms, have complex shapes. Standard indicators may not work well for these. That’s why there’s a rise in customized sterility indicators. These are made to fit specific devices or conditions. They help ensure that even hard-to-reach areas are fully sterilized. Hospitals and device makers are asking for tailored solutions. These custom indicators increase confidence in infection control. As medical tools become more advanced, the need for accurate, device-specific sterilization checks is growing. This trend supports better patient outcomes and reduces the risk of contamination.
Use Cases
Hospitals and Surgical Centers
Sterility indicators are used in every sterilization cycle for surgical tools. This is essential for patient safety. In a medium-sized hospital with 15 operating rooms, over 1,500 instruments may be sterilized each day. That means thousands of indicators are used weekly to confirm effectiveness. Without proper sterilization, the risk of infections increases. Sterility indicators ensure each instrument is safe for use. They also help hospitals meet regulatory guidelines. Staff rely on these indicators to verify every cycle. This regular use supports infection control programs. Hospitals depend on accurate indicators to protect patients during surgeries and other invasive procedures.
Pharmaceutical Manufacturing
Sterility indicators play a critical role in pharmaceutical manufacturing. In cleanrooms, every sterilization cycle must be verified. These environments may run dozens of sterilization cycles each day. Indicators confirm that surfaces, tools, and packaging are germ-free. This is vital before filling or sealing medications. Contamination can spoil entire batches. Using biological indicators ensures that even resistant microbes are killed. Production stops if sterilization is not verified. Therefore, indicators help maintain quality and safety. Manufacturers also use them to comply with strict industry regulations. Without sterility assurance, life-saving medicines could pose serious health risks to patients.
Medical Device Production
Medical device makers use sterility indicators to validate each production batch. For example, a single batch of 10,000 implants must be sterilized before packaging. Biological indicators confirm that sterilization conditions were effective. These are especially important for devices used inside the body. Even the smallest contamination can lead to serious infections. Every step of the production process must meet clean standards. Indicators are used inside sterilizers to detect failures. If one cycle fails, the entire batch may need reprocessing. That’s why reliable sterility indicators are a standard in device manufacturing. They protect patient health and reduce liability.
Laboratory and Research Facilities
Research labs regularly handle infectious materials. Sterility indicators are used to prevent cross-contamination. In a typical microbiology lab, 30 to 40 sterilization cycles are run every week. Each cycle must be validated with indicators. These tools confirm that equipment and waste are properly sterilized. Labs also use them when preparing tools for sensitive experiments. Even a trace of contamination can ruin research. Indicators ensure that lab conditions stay controlled and clean. Staff use them to document every sterilization step. This keeps researchers safe and helps labs meet safety regulations. In high-risk environments, sterility assurance is non-negotiable.
Conclusion
The Sterility Indicators Market is experiencing strong and sustained growth, driven by rising global awareness around infection control and the need for reliable sterilization across healthcare, pharmaceutical, and research settings. With advanced technologies such as rapid-readout indicators, digital integration, and customized solutions for complex medical devices, the market is evolving to meet increasingly strict safety standards. High adoption rates in hospitals, pharmaceutical manufacturing, and labs, coupled with strong regional performance especially in North America and emerging growth in Asia Pacific—underscore the sector’s critical role in modern healthcare. Continued innovation and regulatory support will further fuel market expansion through 2033.


