Table of Contents
Overview
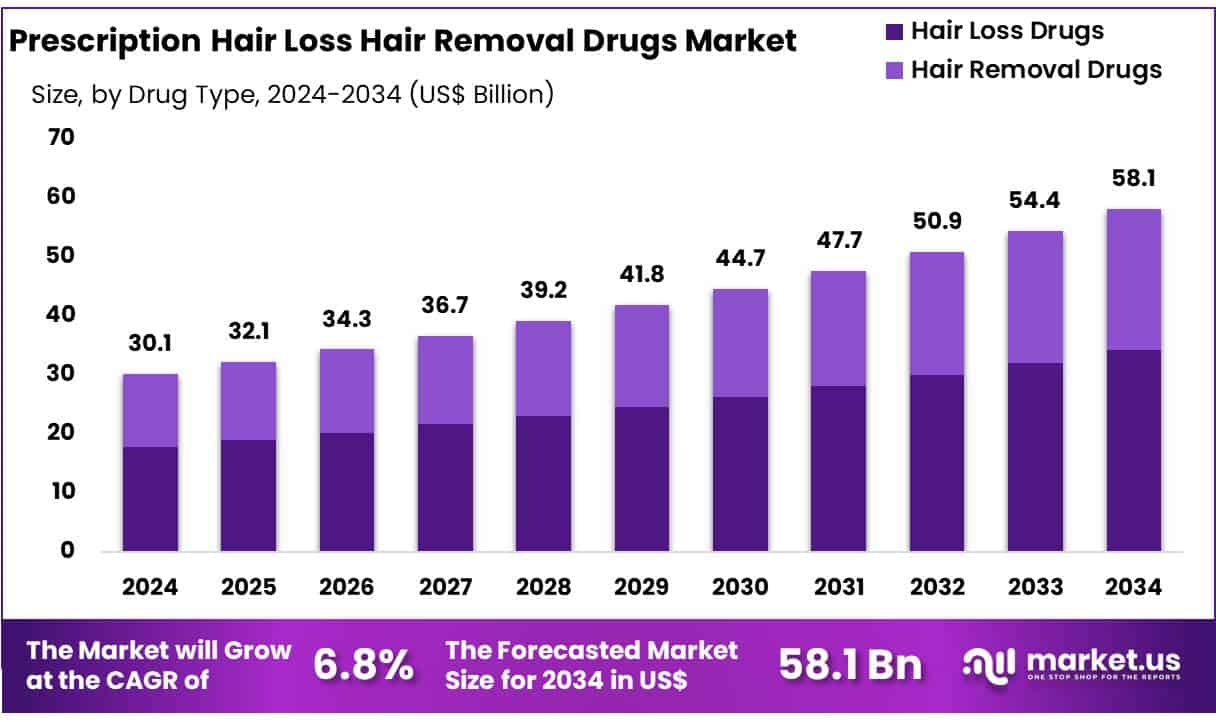
New York, NY – May 27, 2025 – Global Prescription Hair Loss Hair Removal Drugs Market size is expected to be worth around US$ 58.10 billion by 2034 from US$ 30.10 billion in 2024, growing at a CAGR of 6.8% during the forecast period 2025 to 2034.
The global market for prescription drugs targeting hair loss and hair removal is undergoing significant transformation, spurred by advancements in dermatological research, growing awareness regarding aesthetic health, and increased demand for personalized treatments. According to the U.S. National Library of Medicine, androgenetic alopecia affects up to 50% of men and 30% of women globally, reinforcing the clinical and commercial importance of effective hair loss therapies.
Pharmaceutical solutions such as finasteride and minoxidil have shown notable success in slowing hair loss progression, while eflornithine hydrochloride cream remains a leading prescription option for facial hair removal, particularly among women. Increasing consultations with dermatologists and the expansion of telemedicine services have contributed to the rising uptake of prescription-based treatments.
North America continues to lead the market due to a well-established healthcare infrastructure and strong regulatory support for dermatological innovations. However, the Asia-Pacific region is projected to exhibit the fastest growth, driven by increasing cosmetic consciousness and access to dermatological care in urban areas.
With continued R&D investments and regulatory approvals, the market is expected to diversify with novel formulations, combination therapies, and hormone-based solutions. The integration of AI in personalized dermatology is also set to enhance treatment outcomes, improving patient satisfaction and broadening the adoption of prescription therapies for hair loss and removal.
Key Takeaways
- Market Size: In 2024, the global market for Prescription Hair Loss and Hair Removal Drugs generated revenue of USD 10 billion and is projected to grow at a compound annual growth rate (CAGR) of 6.8%, reaching approximately USD 58.10 billion by 2033.
- By Type: The market is categorized into Hair Loss Drugs and Hair Removal Drugs. Among these, Hair Loss Drugs dominated the segment with a 58.9% market share in 2024.
- By Route of Administration: The market is segmented into Topical Formulations, Oral Medications, and Injectables. Topical Formulations emerged as the leading segment, accounting for 48.6% of total revenue.
- By Application: Applications are classified into Chronic Hair Loss and Acute Hair Loss. Chronic Hair Loss remained the dominant category, representing 55.6% of market revenue.
- By Distribution Channel: Distribution is segmented into Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. Retail Pharmacies held the largest share, contributing 45.5% to the overall market.
- By Region: North America led the global market with a 46.8% share in 2024, attributed to strong healthcare infrastructure and increased awareness of prescription dermatology solutions.
Segmentation Analysis
- Drug Type Analysis: Hair Loss Drugs lead the market with a 58.9% share, driven by a high prevalence of androgenic alopecia and proven treatment efficacy. Key drugs include Minoxidil (topical), Finasteride, and Dutasteride (oral), which work by stimulating blood flow or inhibiting DHT. Rising demand for long-term solutions and FDA approvals like Pfizer’s LITFULO (ritlecitinib) in 2023 further support this segment’s rapid growth, making it both the largest and fastest-growing in the market.
- Route of Administration Analysis: Topical Formulations hold a 48.6% share, dominating the market due to ease of use, localized action, and reduced systemic side effects. Products like Minoxidil and Eflornithine (Vaniqa) are widely adopted for their effectiveness and non-invasive application. The segment continues to grow, supported by innovations such as Pelage Pharmaceuticals’ PP405, a novel topical treatment in phase 2 trials, which targets dormant hair follicle stem cells to stimulate regrowth in androgenetic alopecia cases.
- Application Analysis: Chronic Hair Loss is the leading application segment, capturing 55.6% of the market share. This dominance is attributed to the high incidence of long-term hair loss conditions like androgenic alopecia, which require sustained treatment. Drugs such as Minoxidil, Finasteride, and Dutasteride are commonly used to prevent further loss and stimulate new growth, making chronic conditions a more stable and profitable market segment compared to acute or temporary hair loss cases.
- Distribution Channel Analysis: Retail Pharmacies dominate the distribution channel segment with a 45.5% market share, favored for their accessibility, immediate availability, and ability to dispense both over-the-counter and prescription drugs. Consumers prefer retail outlets for their convenience and broad product range. In some regions, certain treatments are available without prescriptions, enhancing accessibility and market penetration, thus reinforcing retail pharmacies as the preferred point-of-sale for hair loss and hair removal medications.
Market Segments
Drug Type
- Hair Loss Drugs
- Minoxidil
- Finasteride
- Dutasteride
- Platelet-Rich Plasma (PRP) Therapy
- Others
- Hair Removal Drugs
- Eflornithine (Vaniqa)
- Laser-based Medications
- Others
Route of Administration
- Topical Formulations
- Oral Medications
- Injectables
Application
- Chronic Hair Loss
- Acute Hair Loss
Distribution channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Regional Analysis
In 2024, North America accounted for 46.8% of the global market share, making it the leading region in the Prescription Hair Loss and Hair Removal Drugs Market. This dominance is driven by high consumer demand for aesthetic treatments and the widespread prevalence of hair loss conditions such as androgenic alopecia. The United States contributes significantly to this growth, supported by advanced healthcare infrastructure, high disposable income, and strong public awareness of available treatments.
Commonly prescribed medications in the region include Minoxidil, Finasteride, and Eflornithine (Vaniqa). The increasing adoption of telemedicine platforms and online pharmacies has improved patient access to prescription-based therapies, enhancing market penetration.
Innovation continues to fuel growth. In July 2024, Sun Pharmaceutical Industries Limited and its affiliates received U.S. FDA approval for LEQSELVI™ (deuruxolitinib) 8 mg tablets, a treatment for severe alopecia areata in adults. This reflects the region’s ongoing advancements in biotechnology and personalized medicine.
While high treatment costs and regulatory compliance remain key challenges, the North American market continues to lead due to its strong innovation pipeline, growing cosmetic focus, and patient-centric approach to hair loss management.
Emerging Trends
- Expansion of JAK Inhibitors for Autoimmune Hair Loss: A third Janus kinase (JAK) inhibitor, deuruxolitinib (Leqselvi™), was approved by the U.S. Food and Drug Administration on July 26, 2024. This oral therapy targets severe alopecia areata by blocking immune attacks on hair follicles. Approximately 700,000 Americans are affected by this condition, and earlier approvals of baricitinib and ritlecitinib have paved the way for deuruxolitinib’s entry into clinical practice.
- Growing Off-Label Use of Oral Minoxidil: Minoxidil, originally approved as a hypertension treatment in the 1970s, is increasingly prescribed in low-dose pill form to manage pattern hair loss. Although not FDA-labeled for hair restoration, convenience of once-daily dosing and direct gut absorption have driven wider off-label adoption. Early reports suggest similar or improved efficacy compared to topical application, especially when supervised by telemedicine providers.
- Regulatory Scrutiny of Topical Formulations: Topical finasteride sprays and solutions have entered the telehealth market despite lacking specific FDA evaluation. A recent advisory highlighted serious side effects—such as depression and sexual dysfunction—linked to these preparations. Heightened regulatory attention is expected to shape prescribing practices and patient counseling going forward.
- Combination and Personalized Therapies: Research is increasingly focused on combining pharmacologic agents with procedural interventions. In one study, adding eflornithine cream to laser hair removal resulted in superior patient-rated outcomes (41.9% favoring combination therapy at six months) compared to laser alone. This trend toward multimodal regimens reflects a move to tailor treatments based on individual response patterns.
- Telemedicine-Driven Access and Monitoring: The rise of telehealth platforms has lowered barriers to prescribing prescription hair-loss drugs. Virtual consultations facilitate ongoing monitoring, dosage adjustments, and adherence support. However, this model has also prompted calls for clearer guidelines to ensure safety, especially for off-label and compounded products.
Use Cases
Management of Male Androgenetic Alopecia
- Drugs Used: Finasteride (1 mg/day) and topical minoxidil (5% solution) are the only FDA-approved prescription treatments for male pattern baldness in the United States.
- Efficacy: Treatment with finasteride has been shown to produce approximately 30 % improvement in hair density after six months of continuous use and to prevent further loss as long as therapy is maintained.
Population Impact: Up to 50 % of men experience clinically significant hair thinning by age 50.
Treatment of Severe Alopecia Areata
- Drug Used: Deuruxolitinib (Leqselvi™), an oral JAK1/JAK2 inhibitor, is indicated for adults with severe alopecia areata.
- Population Impact: An estimated 6.7 million U.S. residents live with alopecia areata, representing about 2 % lifetime risk.
- Clinical Benefit: In pivotal trials, patients receiving deuruxolitinib experienced meaningful scalp hair regrowth, reducing the psychosocial burden of patchy hair loss.
Reduction of Unwanted Facial Hair in Women
- Drug Used: Eflornithine hydrochloride cream (11.5 %) is prescribed to slow facial hair growth in women with hirsutism.
- Efficacy Data: In an open-label study of 25 women, eflornithine cream reduced hair density by 11.4 hairs/cm² at one month and by 16.5 hairs/cm² at two months compared to baseline (p < 0.05).
- Usage Notes: Application twice daily is required indefinitely to maintain benefit, often in conjunction with other hair-removal methods.
Off-Label Oral Minoxidil for Pattern Hair Loss
- Emerging Practice: Dermatologists and primary-care physicians are prescribing low-dose oral minoxidil (0.25–2.5 mg/day) to patients who have difficulty adhering to topical regimens.
- Preliminary Outcomes: Patient surveys report high satisfaction rates due to ease of use; controlled studies are underway to quantify regrowth percentages relative to topical formulas.
Conclusion
The global Prescription Hair Loss and Hair Removal Drugs Market is poised for substantial growth, driven by rising aesthetic awareness, technological innovation, and expanding treatment access through telemedicine. With a projected value of USD 58.10 billion by 2033 and a CAGR of 6.8%, the market is being shaped by the increasing prevalence of chronic hair loss conditions and the adoption of advanced therapies, including JAK inhibitors and off-label oral treatments.
While North America leads due to robust infrastructure and innovation, emerging markets in Asia-Pacific offer strong growth potential, supported by urbanization, personalized care, and evolving regulatory landscapes.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



