Table of Contents
Overview
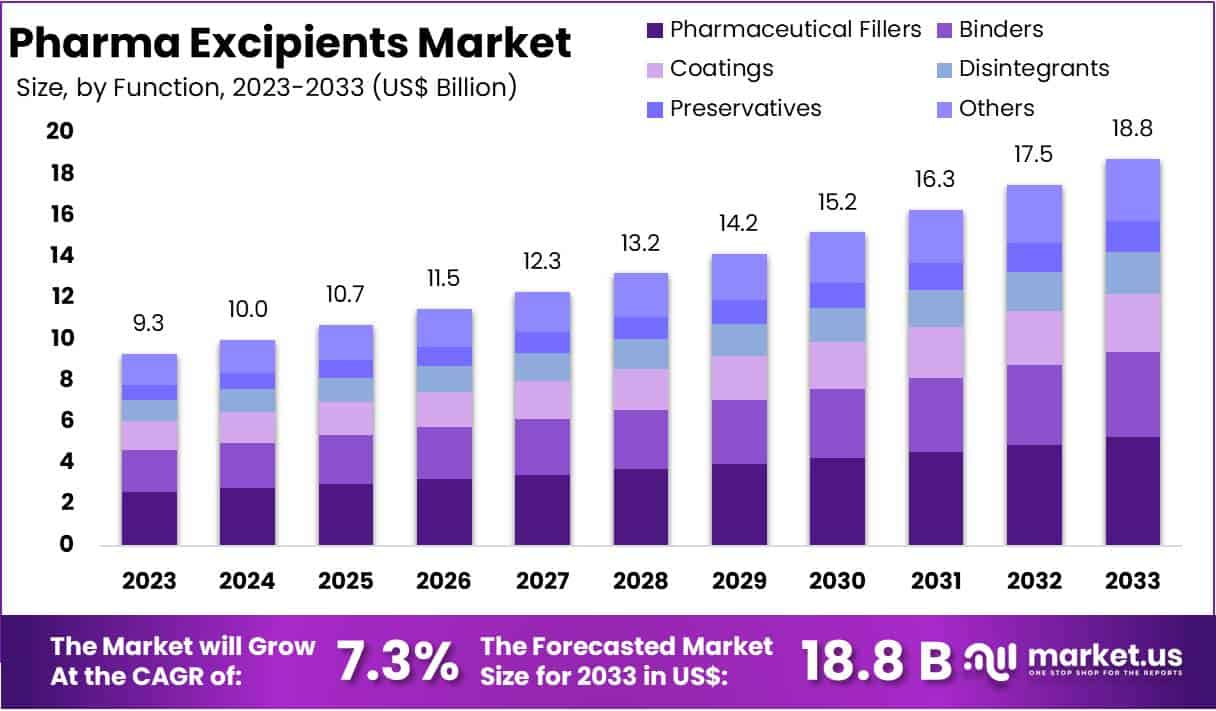
New York, NY – July 24, 2025 – The Global Pharma Excipients Market size is expected to be worth around US$ 18.8 Billion by 2033, from US$ 9.3 Billion in 2023, growing at a CAGR of 7.3% during the forecast period from 2024 to 2033.
The global pharmaceutical excipients market is experiencing consistent growth, driven by the increasing demand for effective drug formulations and the expanding generics sector. Pharmaceutical excipients, which are inactive substances formulated alongside the active ingredient, play a critical role in drug delivery, stability, and bioavailability.
Excipients such as binders, fillers, disintegrants, and coatings are essential in ensuring the efficacy and manufacturability of solid and liquid dosage forms. As pharmaceutical companies continue to innovate in drug development, the need for multifunctional and patient-compliant excipients is rising.
In 2023, solid dosage forms accounted for a significant share of excipient consumption, particularly in tablets and capsules. Polymers and alcohols remain among the most used excipient categories, especially in sustained-release formulations. North America led the market, owing to a strong pharmaceutical manufacturing base and regulatory guidance by the FDA. However, the Asia-Pacific region is emerging as a high-growth area due to cost-efficient production and expanding local demand.
Stringent regulations, growing emphasis on quality standards, and the increasing adoption of novel drug delivery systems are expected to further drive market dynamics. As the pharmaceutical industry evolves toward complex formulations and personalized medicine, the role of innovative excipients is becoming increasingly vital.
Key Takeaways
- The global pharmaceutical excipients market was valued at US$ 9.3 billion in 2023 and is projected to expand to approximately US$ 18.8 billion by 2033, registering a compound annual growth rate (CAGR) of 7.3% during the forecast period.
- In terms of functional segmentation, the pharmaceutical fillers segment emerged as the leading category in 2023, accounting for over 29.4% of the total market share.
- Based on product type, the inorganic chemicals segment dominated the market in 2023, representing more than 60.4% of the overall market share.
- Within the functionality-based application segment, the modified-release excipients held the dominant position, capturing over 31.6% of the market share in 2023.
- Regarding formulation type, the oral formulation segment was the most prevalent in 2023, with a market share exceeding 67.3%, reflecting its wide usage across pharmaceutical products.
- Europe was identified as the largest regional market in 2023, holding a share of more than 38.6%, with a regional market value reaching US$ 3.6 billion.
Segmentation Analysis
- Industrial Advantages: Pharmaceutical excipients offer strategic industrial benefits by reducing production costs, extending shelf life, and improving drug bioavailability. These components enhance formulation stability and support compliance with regulatory standards, accelerating market approvals. They also enable manufacturers to create innovative and patient-centric drug delivery systems, including controlled-release formulations. With growing demand for sustainable manufacturing, green excipients offer a competitive edge, especially in emerging markets. Collaborations with biotech firms further expand opportunities in novel therapeutic areas and biologic drug development.
- Function Analysis: In 2023, pharmaceutical fillers led the function segment, accounting for over 29.4% of the market due to their role in dosage accuracy and manufacturing efficiency. Binders followed, supporting tablet cohesion and stability. Coatings gained traction for drug protection and taste masking. Disintegrants and preservatives maintained consistent use, aiding bioavailability and microbial safety. Together, these functional excipients play an essential role in modern drug formulation, ensuring product effectiveness, quality, and regulatory compliance across a variety of applications.
- Product Analysis: The inorganic chemicals segment dominated the product category in 2023, holding over 60.4% of the market share, driven by their stability and compatibility with active ingredients. Organic chemicals such as oleochemicals and carbohydrates are gaining popularity for their natural origin and multifunctional benefits. Petrochemicals continue to be used for their durability and cost-effectiveness in coatings and binders. Collectively, these product types provide manufacturers with flexible options to optimize formulation performance, stability, and sustainability.
- Functionality Application Analysis: Modified-release excipients captured over 31.6% of the functionality application market in 2023, supporting controlled and sustained drug delivery systems. These excipients improve treatment outcomes and patient adherence by reducing dosing frequency. Solubility and bioavailability enhancers are also in demand, particularly for poorly soluble drugs. Taste masking agents are increasingly used in pediatric and geriatric formulations. Other essential excipients such as stabilizers, binders, and disintegrants continue to support formulation integrity and performance across diverse therapeutic categories.
- Formulation Analysis: The oral formulation segment led the market in 2023, accounting for more than 67.3% of the total share. Oral drugs, including tablets and capsules, are preferred for their convenience, stability, and cost-effectiveness, especially in chronic disease management. Excipients like fillers and disintegrants are vital in these formulations. Topical formulations, including creams and gels, are also gaining momentum for localized treatments. Despite alternative routes, oral formulations are expected to maintain dominance due to strong global patient and provider preference.
Market Segments
By Function
- Pharmaceutical Fillers
- Binders
- Coatings
- Disintegrants
- Preservatives
- Others
By Product
- Organic
- Oleochemicals
- Carbohydrates
- Petrochemicals
- Othe Organic Chemicals
- Inorganic Chemicals
- Calcium Phosphate
- Calcium Carbonate
- Metal Oxides
- Calcium Sulphate
- Other Inorganic Chemicals
By Functionality Application
- Taste Masking
- Stablizers
- Modified-Release
- Solubility & Bioavailablity Enhancement
- Others
By Formulation
- Oral
- Topical
Regional Analysis
In 2023, Europe held a leading position in the global pharmaceutical excipients market, accounting for over 38.6% of the total market share with a valuation of approximately US$ 3.6 billion. This dominance is driven by the region’s robust pharmaceutical manufacturing base and the presence of major industry players actively engaged in the production of both branded and generic drugs.
The region’s stringent regulatory framework, enforced by agencies such as the European Medicines Agency (EMA), ensures high-quality standards for excipient production. These regulatory measures enhance global confidence in European suppliers, positioning the region as a trusted source for pharmaceutical excipients.
Europe’s advanced healthcare infrastructure and growing geriatric population, combined with the increasing prevalence of chronic conditions, continue to sustain demand for pharmaceutical products and, in turn, excipients. The emphasis on patient safety and treatment efficacy further supports the integration of high-performance excipients into drug formulations.
Moreover, ongoing research and development efforts in Europe have led to innovations in excipient technologies, including controlled-release and targeted delivery systems. These advancements are reshaping drug delivery strategies and contributing to improved therapeutic outcomes. As a result, Europe is expected to maintain its leadership in the global excipients market through continued innovation, regulatory strength, and market responsiveness.
Emerging Trends
- Regulatory Support for Novel Excipients: A voluntary FDA pilot program (PRIME) has been established to review excipients that have never been used in approved drug products and are not common in food. In its first two years, CDER planned to accept approximately four initial proposals (two per year) for in-depth review of toxicology and quality data.
- Growth of Co-Processed Excipients: The EMA has released harmonized Q&As defining three risk categories for co-processed excipients (CoPEs) used in solid oral dosage forms. These combined ingredients are increasingly adopted to achieve multiple functions (e.g., binder+disintegrant) in a single material, reducing tablet size and simplifying manufacturing.
- Enhanced Review Capacity for Excipient Suitability Petitions: Under GDUFA performance goals, the FDA aims to review up to 50 excipient suitability petitions within six months in FY 2024, increasing to 70 petitions in FY 2025 and 80 in FY 2026. This rising capacity is intended to accelerate generic drug development when a novel excipient change is proposed.
- Early-Stage Engagement on Excipient Selection: FDA guidance now encourages sponsors to discuss novel excipient use during the IND stage. Early engagement is promoted to address safety and formulation challenges before pivotal clinical studies.
- Replacement of Titanium Dioxide: Following safety evaluations, the EMA issued technical guidance in July 2022 on the removal or replacement of titanium dioxide in oral dosage forms. This has driven research into alternative whitening and opacifying agents compliant with EU food-contact regulations.
Use Cases
- Opioid Abuse-Deterrent Formulations: Two novel excipient proposals aimed at improving viscosity and gel strength for abuse-deterrent opioid tablets were selected in the first two years of FDA’s PRIME program. These excipients are being evaluated for their potential to resist tampering and slow release of active drug.
- Generic Drug Reformulation via Suitability Petitions: In FY 2024, the FDA completed up to 50 excipient suitability petition reviews within the six-month goal. This use case involves generic sponsors petitioning to substitute an FDA-approved excipient (e.g., microcrystalline cellulose) with a functionally equivalent alternative, enabling product launches without full new-drug applications.
- Solid Oral Dosage with Co-Processed Excipients: Manufacturers of immediate-release tablets are increasingly using CoPEs categorized by EMA into Risk Category I (low risk), Category II (moderate risk), and Category III (high risk) based on composition complexity. This approach streamlines formulations by combining two or more functions (e.g., filler+binder) in 1–3 materials.
- IND-Stage Formulation Optimization: During early clinical development, sponsors have leveraged FDA’s recommendation to engage on novel excipients, resulting in improved bioavailability and reduced animal testing. In several IND programs, excipient discussions occurred within 6 weeks of submission to the agency, expediting first-in-human studies.
- Formulation Without Titanium Dioxide: By July 2022, over 30 marketing-authorization holders in the EU had submitted variations to replace titanium dioxide with alternative pigments (e.g., calcium carbonate) in solid oral products, in line with EMA’s technical guidance ([European Medicines Agency.
Conclusion
The global pharmaceutical excipients market is poised for steady expansion, driven by the rising demand for advanced drug formulations, generics, and regulatory reforms supporting excipient innovation. With solid oral dosage forms leading usage and Europe maintaining regional dominance, the market benefits from technological advancements and strong compliance frameworks.
Functional innovation, such as modified-release and bioavailability-enhancing excipients, continues to shape therapeutic strategies. Emerging trends like FDA’s PRIME program, co-processed excipients, and titanium dioxide alternatives underscore the market’s dynamic evolution. As personalized medicine and sustainable manufacturing gain prominence, excipients will remain critical enablers of drug performance, safety, and differentiation in competitive global markets.


