Table of Contents
Overview
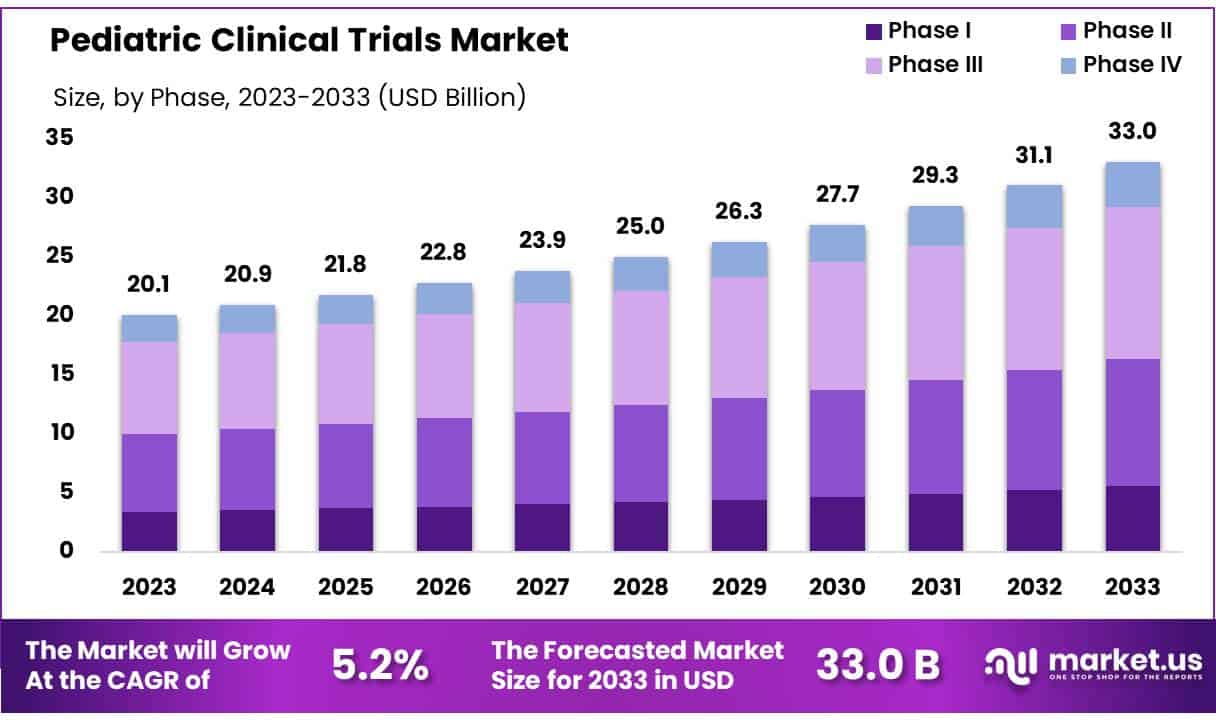
New York, NY – May 21, 2025 – Global Pediatric Clinical Trials Market size is expected to be worth around US$ 33.0 Billion by 2033 from US$ 20.1 Billion in 2023, growing at a CAGR of 5.0% during the forecast period from 2024 to 2033.
The global Pediatric Clinical Trials Market is experiencing steady expansion, driven by the increasing prevalence of chronic and rare diseases among children, along with growing regulatory emphasis on age-appropriate drug development.
Regulatory frameworks such as the U.S. Pediatric Research Equity Act (PREA) and the European Paediatric Regulation have played a pivotal role in encouraging pharmaceutical companies to include pediatric populations in drug trials. Increased R&D investment, expanding global healthcare access, and heightened awareness regarding pediatric-specific therapeutics are further propelling market growth.
Therapeutic areas such as oncology, infectious diseases, and rare genetic disorders represent significant portions of ongoing and planned pediatric trials. North America continues to lead in trial volume due to robust infrastructure and funding support, while the Asia-Pacific region is expected to witness accelerated growth due to rising healthcare investments and patient recruitment capabilities. The pediatric segment remains a critical focus area to ensure safe and effective therapeutics tailored for children, thereby contributing to improved long-term health outcomes globally.
Key Takeaways
- The global Pediatric Clinical Trials market was valued at USD 20.10 billion in 2023 and is anticipated to reach USD 33.04 billion by 2033, growing at a compound annual growth rate (CAGR) of 5.2% during the forecast period.
- Phase III trials accounted for the largest share among all trial phases, contributing 38.9% of the total market revenue.
- Based on study design, Treatment Studies dominated the market, representing 74.2% of the total revenue share.
- By therapeutic indication, the Infectious Diseases segment emerged as the leading contributor, securing a 25.2% share of the global market.
- With regard to sponsorship, industry-sponsored trials led the market in 2023, holding a 52.7% share.
- North America remained the dominant regional market, accounting for the highest revenue share of 39.10% in 2023.
Segmentation Analysis
- Phase Analysis: In 2023, Phase III trials dominated the pediatric clinical trials market, capturing 38.9% of revenue. These trials are essential for confirming the safety and effectiveness of therapies across larger pediatric populations. The segment’s growth is driven by rising demand for targeted treatments. From 2024 to 2033, Phase I trials are expected to expand rapidly due to increased emphasis on early safety assessments, adaptive trial designs, and technological innovations improving trial efficiency in children.
- Study Design Analysis: Treatment studies accounted for the largest market share in 2023, focusing on evaluating therapies specifically for pediatric conditions. These interventional trials are vital for ensuring safe and effective treatments for children, supported by regulatory incentives encouraging pediatric research. Looking ahead, observational studies are expected to grow steadily due to their low cost, quicker timelines, and ethical advantages. Their ability to include diverse populations and generate real-world data enhances their appeal for pediatric research programs.
- Indication Analysis: In 2024, the oncology segment led the market, driven by a rise in pediatric cancer cases and demand for advanced therapies. According to the American Cancer Society, over 9,600 children in the U.S. are expected to be diagnosed with cancer in 2024. This has intensified efforts toward developing pediatric-specific oncology treatments. Meanwhile, the infectious diseases segment is projected to grow rapidly through 2033, propelled by the urgent need for vaccines and therapeutics for conditions like respiratory and gastrointestinal infections.
- Sponsor Analysis: The industry sponsor segment held the largest share in 2023, contributing 52.7% of market revenue. Pharmaceutical and biotech companies continue to invest in pediatric research to meet regulatory requirements and capitalize on incentives such as market exclusivity. These sponsors are embracing modern trial designs, digital technologies, and collaborations with academic institutions to enhance efficiency and compliance. This strategic approach positions industry sponsors as key drivers in advancing safe, effective pediatric therapies globally.
Market Segments
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Treatment Studies
- Observational Studies
By Indication
- Infectious Diseases
- Oncology
- Autoimmune/inflammation
- Respiratory Disorders
- Mental Health Disorders
- Others
By Sponsor
- Industry
- Government Organizations
- Non-Government Organizations
- Associations
- Others
Regional Analysis
In 2023, North America emerged as the leading region, accounting for 39.10% of the global pediatric clinical trials market revenue. This dominance is driven by the presence of established pharmaceutical companies, a high volume of ongoing clinical trials, and a well-developed healthcare infrastructure that supports efficient trial execution.
The United States, in particular, is witnessing strong growth due to substantial investments in research and development from both public and private sectors. Advanced research facilities, specialized pediatric hospitals, and streamlined regulatory frameworks have further enhanced the region’s capacity to conduct large-scale, compliant clinical studies.
Increased government initiatives promoting pediatric research and incentives for pharmaceutical firms have strengthened industry participation. These combined factors firmly position North America as the central hub for pediatric clinical trials, with a solid foundation for sustained expansion in the coming years.
Emerging Trends
- Growth in Medical Device Studies: The number of pediatric medical device (PMD) trials has risen sharply. Between 2014 and 2022, initiation of new PMD trials increased significantly compared with 1999–2013, reaching a total of 1,885 trials out of 12,943 pediatric studies nationally. On average, 1.5 PMD trials were initiated per FDA-Pediatric Device Consortia institution each year during this period.
- Shift toward Precision Medicine: Priority-setting under the Best Pharmaceuticals for Children Act (BPCA) has emphasized precision dosing and targeted approaches. The 2023–2024 BPCA Priority List calls for studies in special populations and recommends leveraging artificial intelligence to inform study design and literature review.
- Expanded Regulatory Support: Since 2003, ten BPCA Priority Lists have guided pediatric research on more than 150 off-patent drugs. To date, 51 clinical trials have been funded under BPCA, 27 clinical study reports have been submitted to the FDA, and 20 pediatric drug labels have been updated based on trial findings.
- Integration of Real-World Evidence: Electronic health record reforms are being promoted to collect regulatory-grade real-world data. This approach is intended to reduce trial burden, speed up evidence generation, and support pragmatic study designs without compromising data quality.
- Focus on Diversity and Inclusion: Recent reviews have documented efforts to enhance racial and ethnic representation in pediatric trials. Greater inclusion is now encouraged at the design stage to ensure that findings are broadly applicable across different child populations.
Use Cases
- Off-Patent Drug Repurposing: Clinical studies are conducted on existing, off-patent medications to establish safe and effective pediatric dosing. Under the BPCA program, 51 such trials have been funded, leading to 20 updates in pediatric labeling for improved dosing and safety information.
- Medical Device Validation: Pediatric medical devices such as child-sized heart valves and insulin delivery pumps have been evaluated in 1,885 PMD trials across U.S. institutions from 1999 to 2022. These studies ensure that device performance and safety are confirmed specifically for children.
- Vaccine Development and Assessment: Trials for pediatric vaccines (e.g., influenza, respiratory syncytial virus) are used to confirm age-appropriate dosing and immune response. In 2015 alone, RSV caused an estimated 33.1 million respiratory infections and 2.7–3.8 million hospital admissions in children under five, underscoring the need for targeted clinical studies.
- Rare Disease and Orphan Drug Studies: The BPCA Priority List has identified ten therapeutic areas including many rare and orphan conditions for focused drug development. Since 2003, over 150 drugs have been prioritized, with trials exploring dosing in neonates, metabolic disorders, and other low-prevalence conditions.
- Optimized Trial Design via AI and Literature Mining: To streamline study planning, two new data streams were integrated in 2022: biannual literature searches by NIH Library specialists and AI-informed searches via the MPRINT Hub. These tools help identify evidence gaps and reduce unnecessary trial complexity.
Conclusion
The global Pediatric Clinical Trials market is witnessing steady growth, supported by increasing prevalence of pediatric diseases, evolving regulatory frameworks, and rising R&D investments. Emphasis on targeted therapies, precision medicine, and ethical trial practices is shaping the future of pediatric research.
North America continues to dominate due to strong infrastructure and policy support, while Asia-Pacific is emerging as a key growth region. Expanding medical device trials, real-world evidence integration, and inclusive trial designs are transforming pediatric research, ensuring safer, more effective treatments tailored for children and contributing significantly to improved global pediatric health outcomes.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



