Table of Contents
Overview
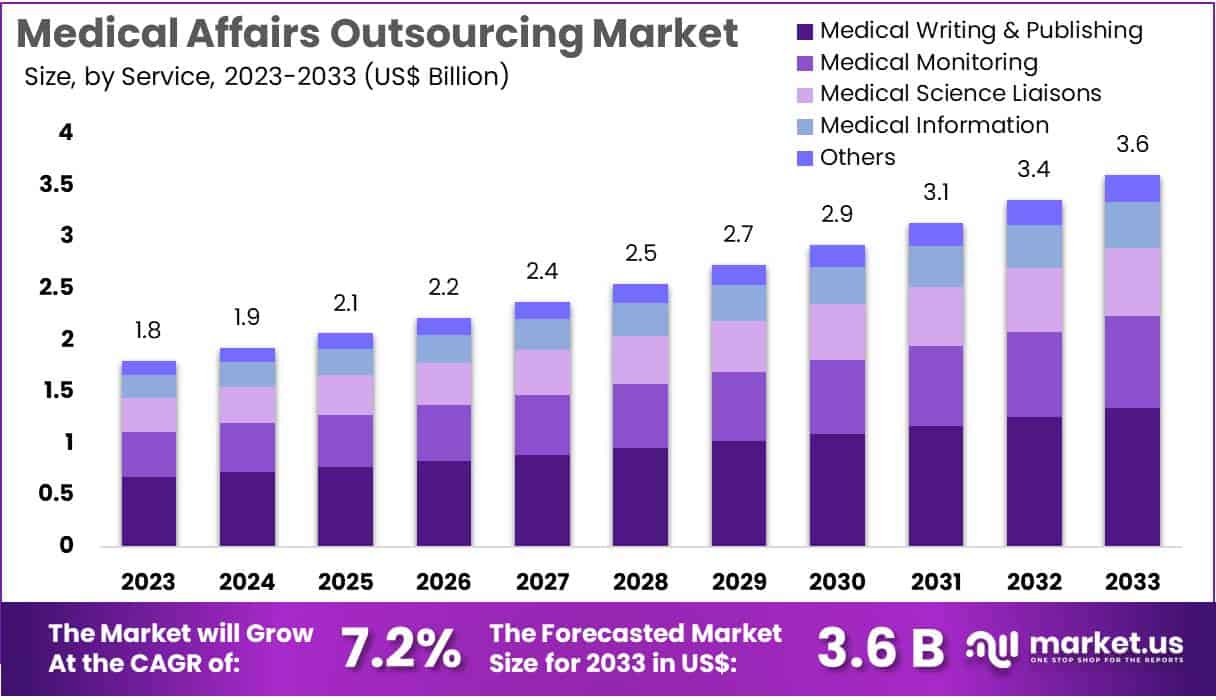
New York, NY – July 18, 2025: The Global Medical Affairs Outsourcing Market is expected to grow from USD 1.8 billion in 2023 to USD 3.6 billion by 2033, at a CAGR of 7.2% between 2024 and 2033. This growth is fueled by rising clinical trials, complex global regulations, and government support for healthcare innovation. As pharmaceutical companies aim to cut costs and stay flexible, outsourcing becomes a valuable solution for managing essential medical tasks without overburdening internal teams.
A major factor driving demand is the increase in global clinical trials. These trials are expanding into emerging regions such as Asia and Latin America. As trial volume grows, so does the need for medical writing, safety monitoring, and communication with healthcare providers. Outsourcing these tasks to specialized firms helps pharmaceutical companies manage this rising workload across various countries while ensuring accuracy and compliance.
Regulatory complexity is another major growth factor. Each country has different rules for documentation and safety data. These frequently changing requirements create challenges for internal teams. Outsourcing firms offer deep expertise in navigating these rules, ensuring regulatory submissions are both accurate and timely. Their global regulatory knowledge helps reduce the risk of delays or non-compliance.
The need to reduce costs and maintain flexibility is also shaping the market. In-house medical affairs teams can be costly to build and maintain, especially for smaller companies. Outsourcing offers access to expert services on demand, allowing companies to scale support based on project needs. This approach keeps operations lean, improves efficiency, and helps businesses stay competitive.
Advancements in digital technologies such as AI, cloud platforms, and real-world data tools are also boosting the demand for outsourced services. These tools make it easier to manage, analyze, and share medical information. Outsourcing partners equipped with these technologies can deliver faster insights, support remote teams, and enhance functions like evidence generation and physician education across global markets.
Finally, government investment in healthcare R&D and the growing focus on patient-centered care are driving outsourcing adoption. Health systems and regulators are increasingly prioritizing real-world outcomes. This shift creates a need for external partners who can collect and report real-world evidence and support data-driven decision-making. Outsourcing enables pharmaceutical companies to focus on drug development while experts handle communication, training, and medical insights.
Key Takeaways
- The medical affairs outsourcing market was valued at approximately US$ 1.8 billion in 2023 and is projected to reach US$ 3.6 billion by 2033, growing at a CAGR of 7.2%.
- Among all service categories, medical writing and publishing emerged as the leading segment in 2023, holding a significant 37.4% share of the market.
- Pharmaceutical companies were the largest end-users, contributing to 48.9% of the total market share during the same year.
- Regionally, North America dominated the market in 2023, accounting for 41.2% of the share, driven by advanced healthcare systems and strong R&D investment.
Segmentation Analysis
Service Analysis
The medical writing and publishing segment dominated the market in 2023 with a 37.4% share. This was mainly due to the rising need for precise and regulation-compliant documentation in healthcare and pharmaceuticals. As regulatory requirements become more complex, companies are turning to external experts for clinical study reports, regulatory filings, and scientific publications. The demand for high-quality manuscripts is also increasing. With clinical trials expanding across new therapeutic areas, the need for outsourced writing services is expected to grow steadily in the coming years.
Industry Analysis
In 2023, the pharmaceutical industry held the largest market share at 48.9%. This reflects the rising demand for expert support in drug development and post-marketing tasks. To cut costs and streamline operations, many companies are outsourcing their medical affairs functions. The growing complexity of global drug regulations is also encouraging this shift. Increased drug approvals, especially in cancer and rare disease areas, are boosting outsourcing activity. The globalization of clinical research and the need for multilingual medical teams further drive this outsourcing trend.
By Service
- Medical Monitoring
- Medical Information
- Medical Writing & Publishing
- Medical Science Liaisons
- Others
By Industry
- Pharmaceutical
- Medical Devices
- Biopharmaceutical
Regional Analysis
North America dominated the medical affairs outsourcing market with a 41.2% share in revenue. This strong performance is linked to increasing regulatory demands and the need for specialized knowledge in managing complex medical data. Companies like Freyr have strengthened the region’s position by offering advanced compliance-focused software. In 2021, Freyr was named among the “10 Best Technology Solution Providers” by USA-9 Technology Magazine. Outsourcing allows pharma and biotech firms to stay compliant while focusing on core activities. Ongoing digital innovation continues to drive growth in the North American market.
Asia Pacific is projected to register the fastest CAGR during the forecast period. The growth is fueled by rising collaborations between global pharmaceutical companies and local institutions. In 2024, Pfizer partnered with NIPER Ahmedabad to support healthcare startups in India through a government-backed accelerator program. This initiative helps innovators bring their ideas to market. Increasing R&D investment and regulatory service demand further support this expansion. Government incentives and improved healthcare infrastructure will continue to push the need for outsourced medical affairs in the region.
Key Players Analysis
The major players in the medical affairs outsourcing market are focusing on innovation and strategic initiatives to boost their competitive edge. They are introducing new services and refining existing ones to meet the growing demands of pharmaceutical and biotech firms. These companies offer specialized support such as medical writing, regulatory consulting, and safety monitoring. By expanding their service portfolios, they provide end-to-end solutions. Their efforts are aligned with the industry’s increasing need for efficiency, cost reduction, and compliance with evolving global healthcare regulations.
To strengthen their market presence, key players are forming alliances with international healthcare organizations. These partnerships enhance trust and broaden their reach across regions. Many companies are also investing in digital platforms and data analytics to deliver faster and more accurate services. By focusing on emerging markets and customizing offerings to meet local regulatory needs, they unlock new opportunities. This approach not only drives market growth but also positions them as reliable partners in a highly regulated and competitive environment.
Emerging Trends
Rise of Specialized Service Providers
Pharmaceutical and biotech companies now prefer to work with niche outsourcing firms. These providers focus only on medical affairs services. They offer specialized support like scientific writing, regulatory documentation, and medical content reviews. Their deep knowledge of the medical field helps ensure high quality and compliance. These firms are not general service providers. Instead, they are experts in handling complex medical tasks. Companies trust them for accurate, clear, and scientific communication. This trend is helping pharma companies save time and get better results. Working with such specialists also supports faster decision-making and improved outcomes across global projects.
Shift Toward Patient-Centric Approaches
Medical affairs is now moving toward patient-centered solutions. Outsourced teams are focusing on collecting real-world data from patients. This helps pharma companies understand how treatments work outside of clinical trials. They can also assess health outcomes more clearly. These insights support value-based care models, where the focus is on patient benefit. Medical affairs providers are helping design programs that address patient needs. They also assist in engaging patients in a meaningful way. This approach improves treatment success and boosts public trust. Companies outsourcing these tasks are now making more informed, patient-driven decisions.
Integrated Outsourcing Partnerships
Earlier, companies hired multiple vendors for different medical tasks. Now, there’s a clear shift toward integrated outsourcing. Pharma companies are forming long-term partnerships with full-service providers. These firms handle everything from writing and reviews to regulatory tasks. A single provider means better communication and fewer handoffs. It also improves coordination and overall project quality. Companies benefit from having a unified strategy and consistent outputs. These integrated partnerships reduce delays and help meet tight deadlines. This model builds trust and results in stronger business relationships. It’s a smarter way to manage complex medical affairs work.
Expansion in Emerging Markets
Outsourcing medical affairs is growing fast in emerging countries. These markets offer skilled professionals at lower costs. Nations like India, the Philippines, and parts of Eastern Europe are becoming key hubs. They deliver high-quality services such as medical writing and data management. Many global companies are now shifting work to these regions. The local talent pool is trained in international standards and regulations. Time zone advantages also help with round-the-clock service. This expansion improves global reach and cost efficiency. Emerging markets now play a big role in supporting the global healthcare system.
Use Cases
Regulatory Documentation
Pharma companies often outsource the preparation of key regulatory documents. These include clinical study reports, investigator brochures, and patient safety summaries. External medical writers ensure that these documents follow strict regulatory guidelines. They help companies meet tight deadlines while maintaining high quality. Outsourcing this task reduces the burden on internal teams and improves accuracy. It also ensures that submissions are ready for review by health authorities like the FDA or EMA. This process supports faster drug approvals and keeps development on track.
Real-World Evidence (RWE) Projects
Many pharma companies outsource Real-World Evidence projects to collect and analyze patient data. These projects look at how drugs work in everyday clinical settings. Outsourced teams gather insights from electronic health records, patient surveys, and treatment databases. This data helps support better healthcare decisions and improves patient outcomes. RWE is often used to justify pricing, show drug effectiveness, or support regulatory submissions. It also helps companies understand the full impact of their products in real life.
Medical Information Services
Outsourcing medical information services helps companies manage inquiries from healthcare professionals, patients, and regulators. Trained professionals provide accurate, science-based responses. They follow approved messaging and company policies to ensure consistency. This service improves communication and supports compliance with regulations. It also allows internal teams to focus on strategy while maintaining quick response times. These experts handle a variety of topics, from drug safety to dosing. Their support helps build trust with external stakeholders and ensures transparency.
Advisory Board Coordination
Organizing scientific advisory boards is often handled by external partners. These experts manage logistics, recruit key opinion leaders, and moderate discussions. Advisory boards provide valuable insights into clinical trial design, treatment gaps, and market strategies. Outsourcing coordination helps companies focus on gathering high-quality feedback. It also ensures meetings run smoothly and follow compliance rules. The input from these panels helps shape scientific direction, marketing plans, and product positioning. This results in more effective and informed decision-making.
Conclusion
In conclusion, the medical affairs outsourcing market is growing steadily due to rising demand for expert support in regulatory tasks, real-world evidence collection, and scientific communication. Pharmaceutical and biotech companies are increasingly relying on specialized service providers to manage complex medical responsibilities. This approach helps reduce internal costs, improve flexibility, and ensure global compliance. Advances in digital tools and strong government backing for healthcare innovation are further boosting market growth. Outsourcing also supports better patient engagement and faster decision-making. As the industry becomes more data-driven and patient-focused, outsourcing will continue to play a key role in helping companies stay competitive and compliant in a rapidly evolving healthcare landscape.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



