Table of Contents
Overview
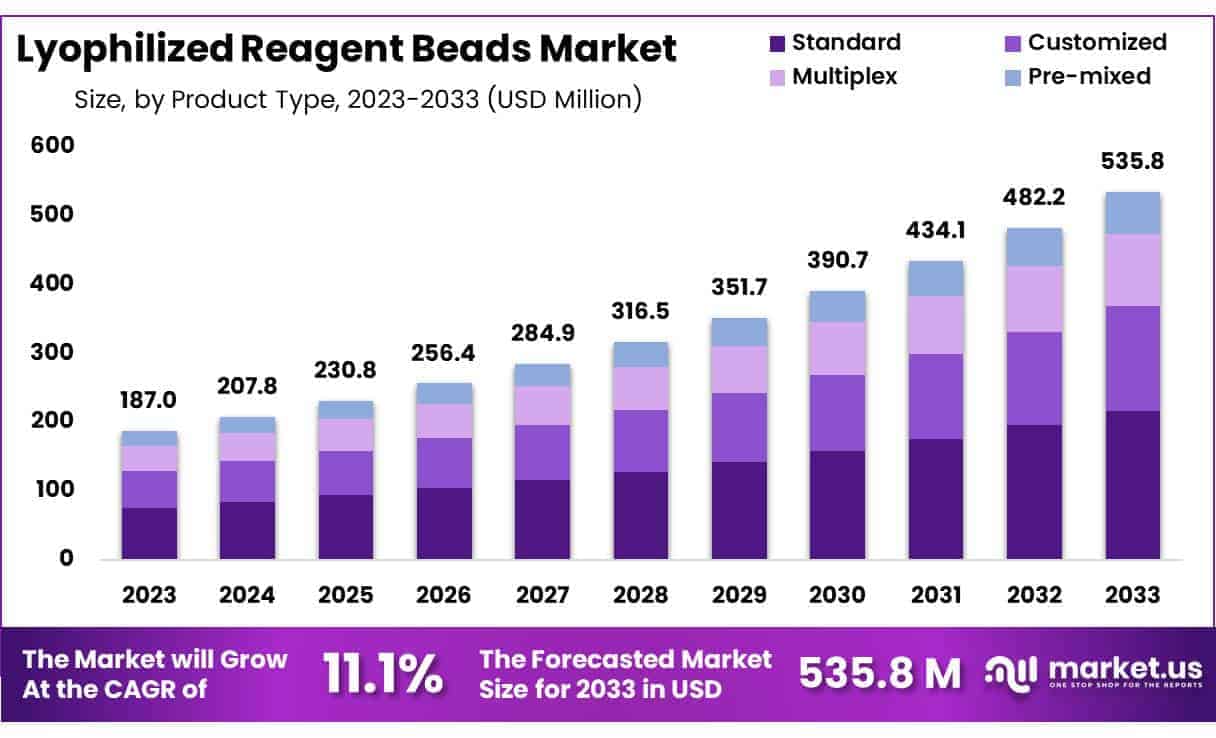
New York, NY – May 13, 2025 – Global Lyophilized Reagent Beads Market size is expected to be worth around US$ 535.8 million by 2033 from US$ 187 million in 2023, growing at a CAGR of 11.1% during the forecast period 2024 to 2033.
Lyophilized reagent beads are defined as freeze-dried, pre-formulated beads containing enzymes, nucleotides, and buffer components. These beads are designed to simplify complex biochemical assays by providing stable, ready-to-use reagents that require only the addition of sample and water. Their enhanced shelf life and ease of handling have been identified as primary factors driving adoption in clinical diagnostics, molecular biology research, and point-of-care testing.
Market growth can be attributed to several key factors. First, the increasing demand for rapid and reliable diagnostic tools has been observed across hospital laboratories and research institutions. Second, the stability of freeze-dried formulations under varied temperature conditions has reduced cold-chain logistics costs. Third, advancements in bead technology have enabled multiplexing capabilities, allowing multiple assays to be conducted simultaneously from a single sample.
Regional analysis indicates that North America will maintain the largest revenue share, supported by advanced healthcare infrastructure and high research spending. Europe and the Asia-Pacific region are also anticipated to exhibit robust growth due to expanding biotech industries and increased government funding for life-science research.
Key Takeaways
- Market Size: The global lyophilized reagent beads market was valued at approximately USD 187 million in 2023 and is projected to reach USD 535.8 million by 2033, reflecting substantial market expansion.
- Market Growth: The market is expected to grow at a robust compound annual growth rate (CAGR) of 11.1% during the forecast period from 2024 to 2033, driven by increasing demand for stable, ready-to-use diagnostic reagents.
- Product Analysis: In 2023, the standard segment accounted for the largest market share of 40.5%, supported by its broad usability in diverse laboratory environments and research applications.
- Application Analysis: Diagnostics emerged as the leading application segment with a 44.8% market share, primarily due to the rising global need for fast, accurate, and stable diagnostic solutions.
- Regional Analysis: North America led the global market in 2023, securing a 40.1% revenue share, attributed to advanced diagnostic infrastructure, high R&D investments, and widespread adoption of molecular testing technologies.
Segmentation Analysis
- Product Type Analysis: In 2023, the standard lyophilized reagent beads segment held a dominant 40.5% market share due to its broad applicability in laboratory environments. These beads offer consistent performance, extended shelf life, and are favored for routine tasks such as PCR and molecular diagnostics. Their seamless integration with automated systems and cost-efficiency makes them highly attractive for diagnostic centers. The continued rise in laboratory automation is expected to further bolster demand for standard formulations in the coming years.
- Application Analysis: The diagnostics segment accounted for 44.8% of the market in 2023, driven by the rising need for accurate and rapid testing solutions. Lyophilized beads enhance reagent stability and enable convenient use in diagnostic kits for infectious diseases, genetic disorders, and point-of-care applications. As healthcare systems adopt portable and time-efficient diagnostic tools, the demand for lyophilized reagents is expected to grow. Expansion of molecular diagnostics across clinical settings will further strengthen this application’s market presence.
Market Segments
By Product Type
- Standard
- Customized
- Multiplex
- Pre-Mixed
By Application
- Diagnostics
- Pharmaceutical & Biotechnology
- Research & Academic Laboratories
- Food & Beverage Testing
Regional Analysis
North America Leads the Lyophilized Reagent Beads Market
In 2023, North America accounted for the largest market share at 40.1%, supported by advancements in molecular diagnostics, biotechnology, and pharmaceutical research. The increasing need for stable, ready-to-use reagents in PCR, qPCR, and next-generation sequencing (NGS) has significantly driven the demand for lyophilized reagent beads across the region.
These beads offer key advantages—such as enhanced reagent stability, minimized contamination risk, and extended shelf life making them highly suitable for diagnostic laboratories and research institutions. The growing incidence of infectious diseases, including COVID-19 and seasonal influenza, has further accelerated their usage in molecular testing. In addition, the rising focus on personalized medicine and companion diagnostics continues to stimulate product innovation and investment, contributing to sustained market expansion.
Asia Pacific to Register the Fastest CAGR
The Asia Pacific region is projected to witness the highest compound annual growth rate (CAGR) during the forecast period, driven by the rapid development of its biotechnology and healthcare sectors. The increasing burden of chronic conditions, such as cancer and diabetes, alongside rising public and private healthcare investments, is expected to boost demand for advanced diagnostic solutions.
Countries like China and India are at the forefront of adopting molecular diagnostic technologies and point-of-care testing, thereby fostering the adoption of lyophilized reagent beads. Growth is further supported by the expansion of research facilities, academic collaborations, and growing awareness of the benefits offered by lyophilized reagents, including ease of use, storage efficiency, and long-term stability.
Emerging Trends
- Enhanced Ambient Stability: The bioactivity of lyophilized reagent beads has been maintained for extended periods without deep freezing. Beads stored at 20 °C retained full activity for almost 30 days, while those kept at 4 °C remained fully active for up to one year. This improvement reduces reliance on ultra-cold storage in many laboratory and field settings.
- Integration into Compact, Single-Vessel Assays: A shift toward all-in-one testing platforms has been observed, where multiple lyophilized beads (e.g., one containing antibiotic at defined concentrations, another containing PCR reagents) are combined in a single tube or microfluidic chamber. Such multiplexed designs enable rapid antibiotic susceptibility tests with minimal manual steps.
- Adoption in Isothermal and Point-of-Care Diagnostics: Lyophilized beads are increasingly used in isothermal amplification devices and point-of-care instruments. For example, all necessary enzymes and primers can be stored in wax-encapsulated beads that release reagents upon heating, eliminating cold-chain needs and simplifying on-site testing procedures.
- Regulatory Standardization of Lyophilization Processes: FDA guidance emphasizes rigorous control of freezing, primary drying, and secondary drying steps to ensure consistent bead quality. This has led manufacturers to adopt standardized protocols and robust in-process monitoring, improving batch-to-batch reproducibility in diagnostic and therapeutic bead products.
- Expansion into Viral and CRISPR-Based Assays: The use of lyophilized reagent beads has grown in advanced viral diagnostics. For instance, Lyo-CRISPR SARS-CoV-2 assays employ beads that only require the addition of 20 µL of water to initiate sensitive detection workflows. This format supports decentralized testing with low resource requirements.
Use Cases
- Rapid Antibiotic Susceptibility Testing: In a 2023 NIH-supported study, two lyophilized beads (one with antibiotic at set concentrations and one with PCR mix) enabled a single-vessel assay for minimum inhibitory concentration (MIC) analysis. The assay provided results in approximately 2 hours with over 95% concordance to standard methods.
- Loop-Mediated Isothermal Amplification (LAMP) for Pathogen Detection: Validation studies demonstrated that LAMP reagent beads stored at 4 °C preserved full enzymatic activity for up to 12 months, compared to just 30 days at 20 °C. This allowed year-round readiness of field-deployable diagnostics targeting bacterial and viral pathogens.
- CRISPR-Cas12 SARS-CoV-2 Assay: A protocol using lyophilized CRISPR-Cas12 beads required only the addition of 20 µL of nuclease-free water. The assay achieved a detection limit as low as 10 copies/µL of viral RNA, supporting rapid, sensitive testing outside traditional laboratories.
- Point-of-Care Platelet Function Testing: The VerifyNow™ PRUTest device contains three lyophilized reagent pellets (ADP pellet, TRAP pellet, and internal control) within a single cartridge. This format enables clinicians to perform platelet aggregation tests at the bedside, with each test completed in under 5 minutes and minimal sample preparation.
Conclusion
The global lyophilized reagent beads market is experiencing robust growth, driven by rising demand for stable, ready-to-use reagents in diagnostics, molecular biology, and point-of-care testing. With a projected CAGR of 11.1% and an expected value of USD 535.8 million by 2033, the market benefits from enhanced reagent stability, automation compatibility, and expanding applications in multiplex and CRISPR-based assays.
North America leads in revenue, while Asia Pacific shows the fastest growth. As technological innovations and regulatory standardization advance, lyophilized beads are poised to play a critical role in the future of decentralized, efficient, and high-performance diagnostic workflows.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



