Table of Contents
Overview
New York, NY – July 22, 2025: The global Hepatitis B Diagnostic Tests Market is set to reach approximately US$ 1,795.3 million by 2033, up from US$ 899.9 million in 2024. This growth reflects a CAGR of 7.15% during 2024 to 2033. The market includes tools for detecting, monitoring, and managing Hepatitis B virus (HBV) infections. Rising infection rates and public health awareness are key drivers. Advancements in diagnostic tools and wider access to healthcare services are further propelling market demand, especially in regions with high HBV prevalence.
Various diagnostic methods are used to detect and manage HBV. These include HBsAg tests to identify active infections and Anti-HBs tests to determine immunity. HBeAg tests check viral replication. Polymerase chain reaction (PCR) tests help quantify viral load, while Liver Function Tests (LFTs) assess liver damage. In 2023, the CDC recommended universal HBV screening for adults aged 18 and above. These testing protocols are critical for early intervention and reducing HBV-related health risks such as liver cancer and cirrhosis.
Despite improved testing methods, HBV remains underdiagnosed and undertreated. The World Health Organization reported 254 million HBV cases in 2022. Yet only 13% were diagnosed and fewer than 3% received antiviral therapy. Co-infections with HIV also present a challenge. About 2.7 million people have both HIV and HBV. The WHO recommends using Tenofovir, a treatment for HIV, which also suppresses HBV. Global health policies are now focusing on integrated screening to improve treatment coverage for both infections.
Regional data show varying patterns in HBV reporting and diagnosis. In 2022, EU/EEA countries logged 28,855 HBV cases, with 47% of unknown status. Acute infections made up just 7% of the total. In the U.S., CDC confirmed 2,126 acute HBV cases. However, actual infections are estimated at around 13,800 due to underreporting and asymptomatic presentations. These numbers highlight the need for better surveillance and public health education to close the gap between actual and reported cases globally.
Innovations are reshaping the HBV diagnostics landscape. Rapid test kits, nucleic acid testing (NAT), and automation are enhancing detection accuracy. Investments in point-of-care (POC) solutions and AI-driven diagnostics are also improving early identification of infections. Government screening programs play a major role in expanding access. However, limited testing access in low-income countries remains a key barrier. Future efforts must prioritize affordability, awareness, and broader diagnostic reach to ensure effective global HBV control and reduce related mortality rates.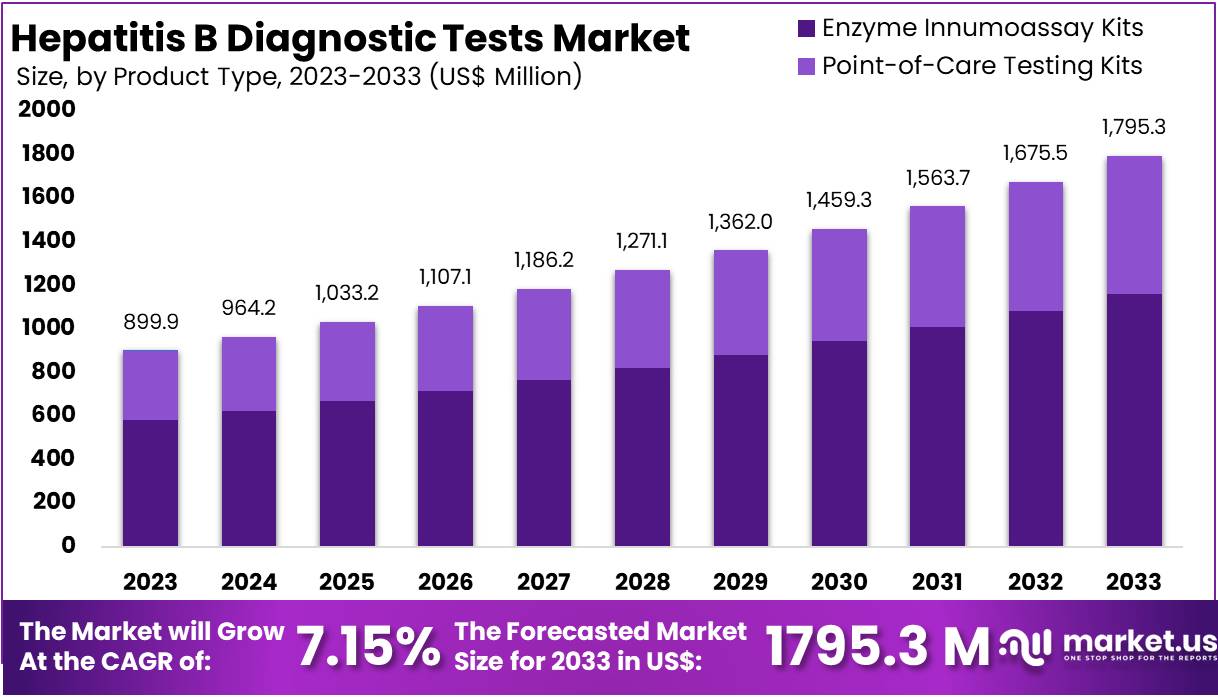
Key Takeaways
- A market analyst noted that the Hepatitis B Diagnostic Tests Market is set to reach US$ 1,795.3 million by 2033, growing steadily at 7.15% CAGR.
- According to experts, Enzyme Immunoassay Kits dominated in 2023, capturing 64.7% market share due to their cost-effectiveness and high diagnostic sensitivity.
- Analysts reported that hospitals led the end-user segment in 2023, holding 42.3% share, thanks to robust diagnostic services and better healthcare infrastructure.
- Industry observers highlighted that North America accounted for 34.30% of the market in 2023, driven by advanced healthcare systems and widespread testing adoption.
- A third-party researcher shared that rising Hepatitis B infection rates and early diagnosis awareness are fueling consistent growth in the diagnostic tests market.
- Observers expect Asia-Pacific to experience the fastest market growth due to rising healthcare investments and increasing Hepatitis B prevalence in the region.
- Point-of-care testing is gaining popularity, offering faster results, shorter hospital stays, and improved patient management in underserved and remote locations.
- Experts say molecular diagnostics are seeing growing demand due to their superior accuracy, aiding in timely detection and effective Hepatitis B treatment.
- Government-led HBV screening programs and global vaccination initiatives are significantly increasing testing rates and driving overall diagnostic test demand.
- New advancements, including AI-powered diagnostics and automated platforms, are enhancing testing speed, accuracy, and overall efficiency in Hepatitis B detection.
Regional Analysis
In 2023, North America emerged as the leading region in the Hepatitis B Diagnostic Tests Market, accounting for over 34.30% of the global share with a market value of US$ 308.67 million. This dominance is attributed to widespread public health awareness and regular screening programs supported by government and private healthcare organizations. These initiatives promote early detection, which significantly increases the demand for diagnostic testing. The region’s advanced healthcare infrastructure, featuring cutting-edge diagnostic technologies, also supports accurate and timely identification of Hepatitis B cases.
Governmental support in the U.S. and Canada further strengthens the market through national vaccination efforts, public health campaigns, and funding for hepatitis B screening. In addition, ongoing innovation from North American healthcare companies plays a vital role. Continuous advancements in diagnostic tools, such as rapid test kits and molecular diagnostics, contribute to improved accuracy and efficiency. These factors collectively position North America as a key driver in the global Hepatitis B Diagnostic Tests Market, maintaining its leadership through a blend of robust infrastructure, policy backing, and technological innovation.
Segmentation Analysis
In 2023, Enzyme Immunoassay Kits dominated the product type segment of the Hepatitis B Diagnostic Tests Market, accounting for over 64.7% of the total share. Their high sensitivity and accuracy make them a preferred choice for early detection of HBV surface antigens. Rapid Diagnostic Tests (RDTs) also held a strong position, offering quick and convenient results, especially in resource-limited and point-of-care settings. Meanwhile, molecular diagnostic tests played a vital role in chronic HBV management, offering precise viral load measurement to guide treatment decisions. Technological advancements continue to enhance the efficiency and accessibility of these diagnostic tools.
In terms of end users, hospitals led the market in 2023 with more than a 42.3% share. Their ability to offer comprehensive testing services, advanced diagnostic tools, and rapid result processing positions them as key hubs for HBV screening and care. Clinics followed closely, providing accessible testing in semi-urban and rural areas, crucial for early detection. Diagnostic centers offer high-precision testing, while home care services meet the needs of patients seeking convenience and privacy. Together, these end-user segments contribute to a more robust and diverse Hepatitis B diagnostic ecosystem.
By Product Type
- Enzyme Innumoassay Kits
- Hepatitis B Surface Antigen Test
- Anti-Hepatitis B Surface Antigen Test
- Anti-Hepatitis B Core Antibody Test
- Point-of-Care Testing Kits
- Strips
- Cassettes/Card
By End User
- Hospitals
- Clinics
- Diagnostic Centers
- Home Care
Key Players Analysis
Bio-Rad Laboratories Inc. stands out as a key player in the Hepatitis B Diagnostic Tests Market, known for its high-quality diagnostic tools and reagents. The company’s products are widely recognized for their accuracy and reliability, which reinforce its strong market position. Similarly, DiaSorin S.p.A. has carved out a competitive edge through its advanced diagnostic technologies. With a strong focus on research and development, DiaSorin continues to enhance its testing capabilities, offering innovative solutions that improve efficiency in Hepatitis B detection.
Abbott Laboratories plays a pivotal role in the global market with its broad portfolio of diagnostic platforms, including immunoassays and molecular tests for Hepatitis B. Its continuous innovation and dependable solutions support widespread adoption across various healthcare systems. Other notable contributors like Meridian Bioscience and bioMérieux SA Inc. are also driving market growth. Meridian specializes in rapid, easy-to-use testing kits, expanding access in diverse clinical settings. Meanwhile, bioMérieux focuses on early detection technologies, strengthening its role in disease management. Collectively, these companies contribute to innovation and increased global accessibility in Hepatitis B diagnostics.
Leading Market Key Players
- Bio-Rad Laboratories Inc.
- DiaSorin S.p.A.
- Abbott Laboratories
- Meridian Bioscience
- bioMérieux SA Inc.
- Vista Diagnostics International
- Biogate Laboratories Ltd.
- J.Mitra & Co. Ltd.
- Siemens Medical Solutions USA Inc.
- General Biologicals Corporation
Emerging Trends
Rise of Point-of-Care Testing
Point-of-care testing (POCT) is becoming more common in Hepatitis B diagnosis. These rapid tests give results within minutes and are easy to use. They are especially helpful in rural or low-resource areas where lab access is limited. Healthcare workers can test patients on-site, avoiding delays. POCT also reduces patient anxiety by offering immediate answers. Many health organizations now prefer these quick kits for mass screenings and urgent testing needs. As testing becomes more decentralized, the demand for POCT will likely grow. It supports early detection and faster treatment decisions, improving patient outcomes overall.
Integration of AI in Diagnostics
Artificial Intelligence (AI) is changing the way Hepatitis B is diagnosed. AI helps read complex test results quickly and with more accuracy. It can detect patterns in test data that humans might miss. This reduces diagnostic errors and speeds up the process. AI is being used in both hospital labs and point-of-care settings. It supports lab technicians by automating result interpretation. Some AI platforms also help doctors decide on treatment plans. As AI tools become more affordable, they will likely become a standard part of diagnostic workflows. This trend improves both testing quality and efficiency.
Focus on Early Screening Programs
Governments and health organizations are encouraging early screening for Hepatitis B. Many countries now recommend that all adults, especially those over 18, get tested at least once. Early screening helps detect the virus before symptoms appear. This prevents the disease from spreading and reduces the risk of liver damage. Hospitals and clinics are adding routine HBV tests to general health check-ups. Educational campaigns are also being used to raise public awareness. With better screening, more people can receive timely treatment. This trend supports global efforts to control and eliminate Hepatitis B infections.
Combination Testing for Multiple Viruses
Combination test kits are gaining popularity in diagnostics. These kits can detect Hepatitis B, Hepatitis C, and HIV in one go. This saves time for both patients and healthcare workers. It also reduces the cost of testing and limits the need for multiple blood samples. Clinics and hospitals use these tests in routine check-ups, especially for high-risk groups. With one sample, multiple diseases can be ruled out or diagnosed. This approach improves patient care and boosts testing efficiency. As technology advances, more combo tests are expected to enter the market.
Self-Testing Kits for Home Use
At-home testing for Hepatitis B is an emerging trend. Similar to pregnancy tests, these kits are easy to use and offer quick results. Users can test themselves privately without visiting a clinic. This is especially helpful for people in remote areas or those who want privacy. Home test kits often come with simple instructions and digital support. Some link to mobile apps that guide users or connect them with doctors. While still new, self-testing could play a major role in early detection. It encourages more people to get tested and take control of their health.
Mobile Health Solutions
Mobile health, or mHealth, is becoming part of Hepatitis B diagnostics. Some healthcare apps now offer result tracking, doctor consultations, and reminders for follow-ups. Patients can receive guidance immediately after testing positive. These apps are especially useful in places with limited healthcare access. They also help patients manage appointments and medication schedules. By combining test results with mobile technology, care becomes faster and more connected. This trend supports better disease management and encourages regular screening. As smartphones become more widespread, mobile health tools will play a larger role in Hepatitis B care.
Use Cases
Hospital-Based Early Detection Programs
Hospitals play a major role in screening for Hepatitis B. Before any major surgery or blood transfusion, patients are routinely tested for HBV. In large urban hospitals, over 500 tests can be conducted each week for surgical clearance alone. Early detection helps protect both the patient and medical staff. Testing also reduces the risk of spreading infection during medical procedures. With advanced facilities and trained staff, hospitals ensure accurate diagnosis. This approach supports timely treatment and lowers complications. Hospitals remain one of the most reliable places for large-scale Hepatitis B screening and follow-up.
Pregnancy Screening in Maternity Clinics
Pregnant women are routinely tested for Hepatitis B during prenatal visits. This prevents mother-to-child transmission, which is one of the leading ways HBV spreads. In high-traffic maternity clinics, more than 50 Hepatitis B tests are done each day. If a pregnant woman tests positive, treatment and newborn vaccination can begin immediately. This early step is critical in reducing chronic infections in infants. Maternity clinics are well-equipped for such screenings and follow strict guidelines. Regular testing during pregnancy has become a vital part of maternal and child healthcare services worldwide.
Blood Donation Centers and Safety Checks
Blood banks screen every donated unit for Hepatitis B to ensure safe transfusions. This testing prevents infected blood from being used in hospitals and clinics. A single blood donation center may process and test over 1,000 blood units each month. Screening includes both surface antigen and molecular tests for added accuracy. Any blood found to be infected is discarded, and donors are notified. These centers follow strict safety protocols to maintain public trust. Regular testing helps keep the blood supply safe and supports national health goals to reduce HBV transmission.
Community Health Camps in Rural Areas
In rural regions, access to testing is limited. Mobile health camps help bridge that gap. These camps offer free Hepatitis B testing and reach people who rarely visit doctors. A single health camp may screen 200 to 300 individuals in a day. Early identification helps start treatment before symptoms worsen. These camps often work in partnership with local governments and NGOs. People who test positive are referred to nearby hospitals or clinics. Such community-based testing plays a key role in early detection and awareness, especially in low-resource settings.
Testing in High-Risk Populations
High-risk groups include prisoners, people in rehab centers, and migrant workers. These groups are more vulnerable to Hepatitis B due to lifestyle factors and limited healthcare access. Regular testing in such environments helps control the spread of infection. For example, out of 1,000 tested individuals in these settings, 80 to 100 may test positive. Early diagnosis allows for isolation and timely treatment. Testing also helps health authorities monitor infection patterns. These programs are often supported by public health departments or NGOs focusing on disease control.
Corporate Wellness Programs
Some companies now include Hepatitis B testing in their employee wellness packages. This is common in industries with large workforces, such as manufacturing or IT. In a company with 5,000 employees, regular screening can detect undiagnosed cases. Early detection prevents outbreaks and improves workplace health. Testing may be done during annual health check-ups or wellness drives. Employers benefit from healthier staff and reduced sick leave. These programs also promote awareness and encourage vaccination. Corporate testing is a growing trend in occupational health and preventive care.
FAQs Hepatitis B Diagnostic Tests
1. What are Hepatitis B diagnostic tests?
Ans:- Hepatitis B diagnostic tests are medical tests used to detect the presence of the Hepatitis B virus (HBV), measure the level of infection, and monitor disease progression or treatment.
2. What are the common types of Hepatitis B tests?
- HBsAg Test – Detects active infection.
- Anti-HBs Test – Confirms immunity or recovery.
- HBeAg Test – Indicates active virus replication.
- HBV DNA Test (PCR) – Measures viral load.
- Liver Function Tests (LFTs) – Checks liver damage.
3. Who should get tested for Hepatitis B?
Ans:- Adults aged 18 or older, pregnant women, individuals with high-risk behaviors, healthcare workers, and those born in regions with high HBV prevalence should get tested.
4. Is Hepatitis B testing available at home?
Ans:- Yes, some companies now offer FDA-approved self-test kits. These are easy to use and offer privacy, though follow-up at a clinic may still be necessary.
5. How accurate are Hepatitis B tests?
Ans:- Most modern diagnostic tests are highly accurate, with sensitivity and specificity rates exceeding 95%. Molecular tests (PCR) are considered the gold standard for confirmation.
6. What is driving the growth of the Hepatitis B Diagnostic Tests Market?
Ans:- Key drivers include rising HBV infection rates, early screening initiatives, improved diagnostic technologies, and greater awareness among healthcare providers and the public.
7. Which regions lead the market?
Ans:- North America holds a leading position due to its advanced healthcare infrastructure and regular screening programs. Asia-Pacific is expected to grow fastest due to high HBV prevalence.
8. What product types dominate the market?
Ans:- Enzyme Immunoassay Kits dominate due to their high sensitivity and cost-efficiency. Molecular diagnostics are gaining traction for their accuracy in measuring viral load.
9. Who are the key players in this market?
Ans:- Major companies include Bio-Rad Laboratories, DiaSorin S.p.A., Abbott Laboratories, bioMérieux SA, and Meridian Bioscience. These players invest heavily in innovation and global distribution.
10. What are the main challenges facing the market?
Ans:- Challenges include limited access in low-income regions, lack of awareness, underdiagnosis, and high costs of molecular tests in some countries.
Conclusion
The Hepatitis B Diagnostic Tests Market is on a strong growth path, projected to reach approximately US$ 1,795.3 million by 2033, driven by a CAGR of 7.15%. This expansion is fueled by rising global infection rates, greater awareness, and improved access to advanced diagnostics such as enzyme immunoassays and molecular tests. While North America currently leads the market due to its robust healthcare systems, Asia-Pacific is expected to see the fastest growth thanks to increased healthcare investments. Innovations like AI-powered tools, point-of-care testing, and mobile health solutions are reshaping detection methods, making testing faster and more accessible. Continued emphasis on early screening, self-testing kits, and public health initiatives will be key to closing the diagnosis and treatment gap, especially in underserved regions.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



