Table of Contents
Overview
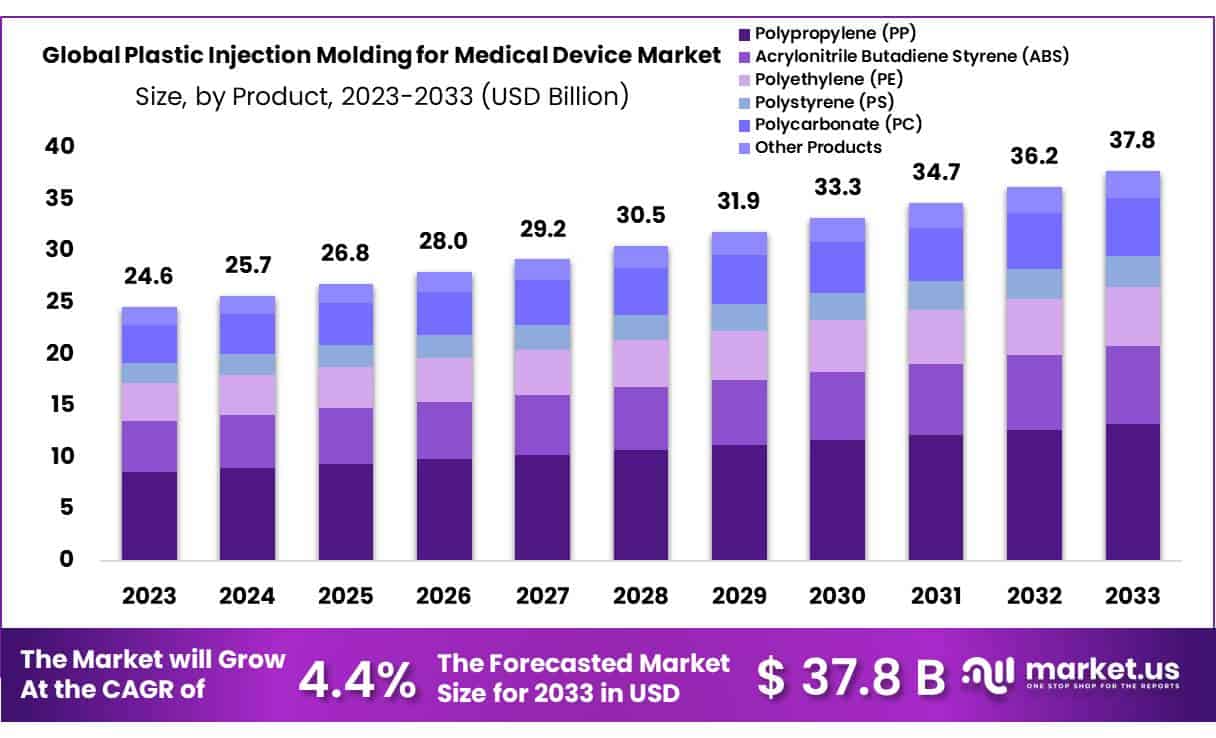
New York, NY – Jan 29, 2026 – The Global Plastic Injection Molding for Medical Device Market size is expected to be worth around USD 37.8 Billion by 2033 from USD 24.6 Billion in 2023, growing at a CAGR of 4.4% during the forecast period from 2024 to 2033.
Plastic injection molding is increasingly recognized as a foundational manufacturing process in the medical device industry, driven by its ability to deliver precision, consistency, and scalability. The process involves injecting molten medical-grade polymers into a precision-engineered mold, where the material is cooled and solidified into a final component with exact specifications.
The adoption of plastic injection molding for medical devices can be attributed to its compatibility with strict regulatory and quality requirements. Medical-grade plastics used in this process are designed to meet standards for biocompatibility, chemical resistance, and sterilization, making them suitable for applications such as syringes, diagnostic components, surgical instruments, and drug-delivery devices.
From a production perspective, plastic injection molding supports high-volume manufacturing while maintaining tight tolerances and repeatability. This consistency is critical for medical applications, where even minor dimensional variations can impact performance and patient safety. In addition, the process allows for complex geometries and integrated features, reducing the need for secondary assembly operations and improving overall efficiency.
Cost efficiency is another key advantage. Once molds are developed, large-scale production can be achieved with relatively low per-unit costs, supporting both established medical device manufacturers and emerging healthcare technology companies. Automation capabilities further enhance production reliability and reduce the risk of contamination in controlled manufacturing environments.
As healthcare demand continues to expand globally, plastic injection molding is expected to remain a strategic manufacturing solution, supporting innovation, regulatory compliance, and scalable production across a broad range of medical device applications.
Key Takeaways
- Market Size: The Plastic Injection Molding for Medical Device market is projected to reach approximately USD 37.8 billion by 2033, rising from USD 24.6 billion in 2023, reflecting steady long-term expansion.
- Market Growth: Market growth is anticipated at a compound annual growth rate (CAGR) of 4.4% over the forecast period spanning 2024 to 2033, supported by increasing demand for medical-grade plastic components.
- Product Analysis: Polypropylene (PP) has emerged as the leading material segment, accounting for 35.1% of the market share, driven by its versatility, cost efficiency, and suitability for medical applications.
- Application Analysis: Within the application landscape, medical components represent the largest segment, capturing 25.2% of the total market share, due to their widespread use across diagnostic, surgical, and therapeutic devices.
- Regional Analysis: North America continues to dominate the global market, holding 39.5% market share in 2023, supported by advanced healthcare infrastructure and strong medical device manufacturing capabilities.
Regional Analysis
On the basis of region, the market is segmented into North America, Europe, Asia Pacific, South America and Middle East & Africa. North America accounts for the majority share dominate 39.5% market share in 2023 in the global plastic injection molding for the medical device market, followed by Europe.
Emerging economies, such as China and India, are expected to register the highest growth rate over the forecast period. Countries and regions such as South America & Middle East & Africa are expected to register stable growth over the forecast period.
Emerging Trends
- Advanced Materials: The adoption of high-performance polymers such as Polyetheretherketone (PEEK) and Polyetherimide (PEI) is increasing. These materials offer enhanced mechanical strength, thermal stability, and biocompatibility, making them suitable for demanding medical and surgical applications.
- Micro-Injection Molding: Micro-injection molding is gaining prominence due to its ability to manufacture ultra-small, highly precise components. This capability is essential for microfluidic devices, miniature sensors, and minimally invasive medical technologies requiring complex geometries and tight tolerances.
- Overmolding and Insert Molding: Overmolding and insert molding techniques enable the integration of multiple materials or embedded components, such as metals, within a single part. These processes improve product functionality, reduce secondary assembly operations, and enhance overall manufacturing efficiency.
- Automation and Industry 4.0 Integration: The integration of automation and Industry 4.0 technologies, including IoT-enabled systems and artificial intelligence, is transforming medical injection molding. These technologies support real-time process monitoring, predictive maintenance, and enhanced quality assurance across production lines.
- Sustainability Practices: Sustainability is becoming a strategic focus, with manufacturers increasingly using recyclable or bio-based polymers and optimizing energy consumption. These practices help reduce environmental impact while ensuring compliance with evolving regulatory and sustainability standards.
Use Cases
- Syringes and Needles: Plastic injection molding is widely used for the mass production of syringes and needles, with facilities manufacturing more than 50 million units per month, ensuring consistent quality, precision, and compliance for single-use medical applications.
- Implantable Components: The technology is extensively applied in producing implantable components for orthopedic and surgical procedures. Injection-molded parts provide high durability, dimensional accuracy, and biocompatibility required for long-term implantation.
- Diagnostic Equipment: Injection molding supports the production of diagnostic consumables such as test tubes, petri dishes, and sample containers, enabling high-volume, sterile, and cost-effective manufacturing for laboratory and diagnostic workflows.
- Drug Delivery Systems: Components for drug delivery devices, including inhalers and nebulizers, are manufactured using injection molding to ensure precise dosing, consistent performance, and reliability in both acute and chronic treatment applications.
- Orthopedic Devices: Injection molding is used to manufacture orthopedic braces and support devices, offering lightweight structures, mechanical strength, and improved patient comfort while allowing scalable and cost-efficient production.
Frequently Asked Questions on Plastic Injection Molding for Medical Device
- Which plastics are commonly used in medical injection molding?
Medical injection molding typically uses biocompatible polymers such as polypropylene, polyethylene, polycarbonate, PEEK, and ABS, as these materials offer chemical resistance, durability, sterilization compatibility, and compliance with international medical safety standards. - Why is injection molding preferred for medical device manufacturing?
Injection molding is preferred due to its ability to deliver high-volume production with tight tolerances, repeatability, minimal material waste, and cost efficiency, making it suitable for both disposable and reusable medical components. - How is regulatory compliance ensured in medical injection molding?
Regulatory compliance is ensured through validated processes, cleanroom manufacturing, material traceability, and adherence to standards such as ISO 13485 and FDA guidelines, ensuring patient safety and consistent product performance. - What factors are driving the plastic injection molding for medical device market?
Market growth is driven by rising healthcare demand, increasing use of disposable medical devices, technological advancements in polymers, and the growing preference for cost-effective, high-precision manufacturing solutions in medical applications. - How large-scale production impacts the medical injection molding market?
Large-scale production enables manufacturers to reduce per-unit costs, improve supply chain efficiency, and meet growing global demand for medical devices, particularly in high-volume consumables such as syringes and diagnostic components. - Which regions dominate the medical injection molding market?
North America and Europe dominate due to advanced healthcare infrastructure and regulatory frameworks, while Asia-Pacific is experiencing rapid growth, supported by expanding medical manufacturing capacity and increasing healthcare investments. - How does technological innovation influence market growth?
Technological innovation, including micro-molding, automation, and advanced tooling, enhances precision and production efficiency, enabling manufacturers to meet complex medical design requirements and support the development of next-generation medical devices.
Conclusion
Plastic injection molding remains a critical manufacturing technology for the medical device industry, offering precision, scalability, and regulatory compliance. Its ability to support high-volume production with consistent quality makes it well suited for applications ranging from syringes and diagnostic components to implantable and drug-delivery devices.
Market growth is being supported by rising healthcare demand, advancements in medical-grade polymers, automation, and micro-molding technologies. Regional leadership by North America, alongside rapid expansion in Asia-Pacific, further strengthens market momentum. Overall, plastic injection molding is expected to continue enabling cost-efficient, innovative, and reliable medical device production globally.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



