Table of Contents
Overview
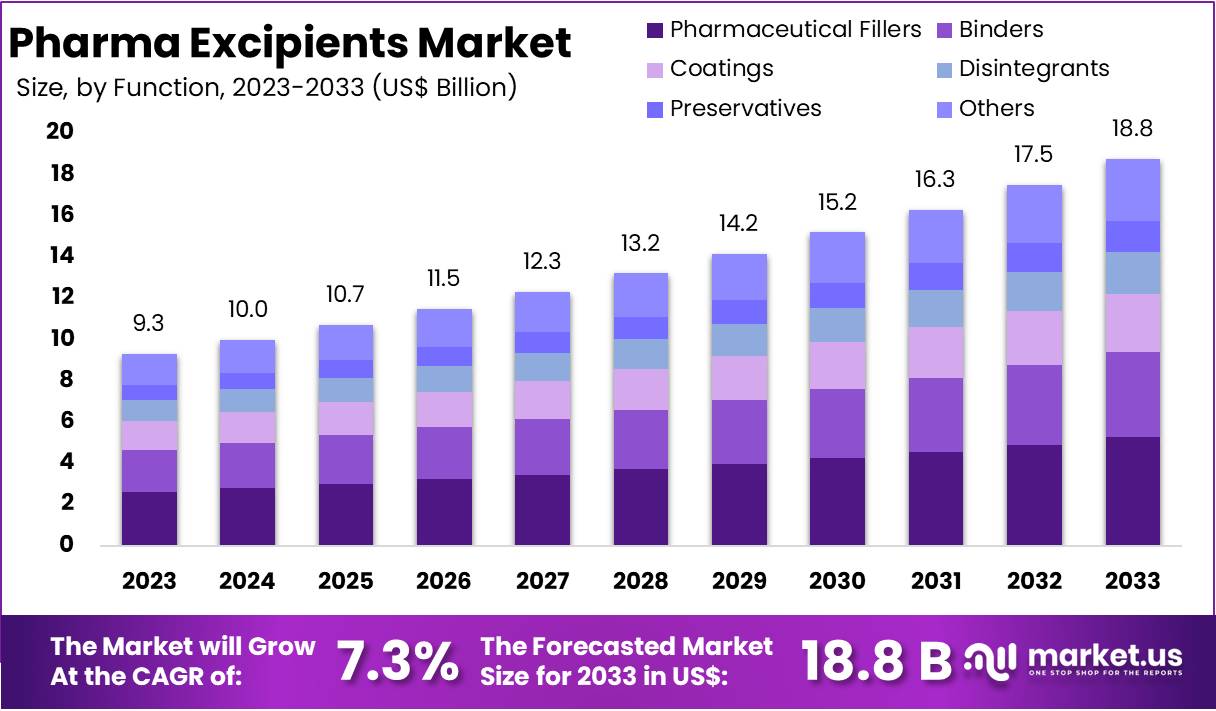
New York, NY – Feb 17, 2026 – The Global Pharma Excipients Market size is expected to be worth around US$ 18.8 Billion by 2033, from US$ 9.3 Billion in 2023, growing at a CAGR of 7.3% during the forecast period from 2024 to 2033.
The global pharmaceutical excipients market is witnessing steady expansion, supported by the rising demand for advanced drug formulations and improved delivery systems. Pharmaceutical excipients, defined as inactive substances formulated alongside active pharmaceutical ingredients (APIs), play a critical role in ensuring stability, bioavailability, safety, and patient compliance. These components are widely utilized in tablets, capsules, injectables, topical formulations, and novel drug delivery platforms.
Market growth is being driven by increasing pharmaceutical production, a growing prevalence of chronic diseases, and expanding investments in research and development activities. The shift toward biologics and personalized medicines has further increased the need for high-performance excipients with enhanced functionality. In addition, regulatory emphasis on product quality and safety standards has encouraged manufacturers to adopt multifunctional and co-processed excipients.
Solid dosage forms continue to account for a significant share of excipient consumption, primarily due to ease of administration and cost efficiency. Key product categories include binders, fillers, disintegrants, coating agents, and lubricants. Among these, functional excipients with improved solubility and controlled-release properties are experiencing higher demand.
Regionally, North America and Europe maintain established market positions due to advanced pharmaceutical infrastructure, while Asia-Pacific is emerging as a high-growth region driven by expanding manufacturing capacity and favorable regulatory frameworks.
Overall, the pharmaceutical excipients market is expected to register sustained growth, supported by innovation in formulation technologies and increasing global healthcare expenditure.
Key Takeaways
- The global Pharma Excipients Market is anticipated to attain a valuation of US$ 18.8 billion by 2033, expanding at a CAGR of 7.3% from US$ 9.3 billion recorded in 2023.
- In 2023, the Pharmaceutical Fillers segment emerged as the leading category within the function segment, accounting for over 29.4% of the total market share.
- The Inorganic Chemicals segment dominated the product category in 2023, contributing more than 60.4% to the overall Pharma Excipients Market share.
- Within the functionality application segment, the Modified-Release category secured a market share exceeding 31.6% in 2023.
- The Oral formulation segment held the dominant position in 2023, representing more than 67.3% of the total share in the formulation segment.
- Europe accounted for the largest regional share in 2023, capturing over 38.6% of the global market, with a valuation of US$ 3.6 billion.
Regional Analysis
In 2023, Europe accounted for over 38.6% of the global pharmaceutical excipients market, reaching a value of US$ 3.6 billion. This leadership position has been supported by a well-established pharmaceutical manufacturing base and the presence of several globally recognized drug manufacturers. Increased production of generic medicines and sustained investments in research and development activities have significantly strengthened regional demand for high-quality excipients.
A stringent regulatory framework has further reinforced Europe’s position in the global market. Strict compliance requirements and high quality standards have ensured superior safety, consistency, and performance of excipients, enhancing global confidence in European suppliers.
Moreover, advanced healthcare infrastructure, rising healthcare expenditure, an aging population, and increasing prevalence of chronic diseases have consistently driven pharmaceutical consumption. Continuous innovation in advanced and controlled-release excipients has also supported formulation improvements, reinforcing Europe’s dominance in the global pharmaceutical excipients industry.
Emerging Trends in Pharmaceutical Excipients
- Regulatory Initiatives Supporting Novel Excipients: A voluntary pilot initiative, known as the U.S. Food and Drug Administration PRIME program, has been introduced to facilitate the evaluation of excipients not previously incorporated in approved drug products and not widely used in food applications. Within its initial two-year implementation phase, the Center for Drug Evaluation and Research (CDER) planned to assess approximately four proposals (around two annually). The program focuses on detailed review of toxicological profiles and quality data to strengthen confidence in innovative excipient adoption.
- Expansion of Co-Processed Excipients (CoPEs): The European Medicines Agency has issued harmonized guidance outlining three defined risk categories for co-processed excipients used in solid oral dosage forms. These materials integrate multiple functional attributes such as binding and disintegration within a single composite excipient. Their increasing utilization is enabling tablet size reduction, enhanced manufacturing efficiency, and improved formulation robustness.
- Strengthened Review Capacity for Excipient Suitability Petitions: Under Generic Drug User Fee Amendments (GDUFA) performance commitments, the FDA has expanded its capacity to evaluate excipient suitability petitions. The agency targeted the review of up to 50 petitions within a six-month timeframe in FY 2024, with projected increases to 70 in FY 2025 and 80 in FY 2026. This enhanced throughput is expected to accelerate generic drug approvals where excipient modifications are proposed.
- Early Regulatory Dialogue on Excipient Strategy: Recent FDA guidance encourages pharmaceutical sponsors to initiate discussions on novel excipient incorporation during the Investigational New Drug (IND) phase. Early engagement enables proactive identification of safety considerations and formulation challenges, thereby reducing regulatory uncertainty prior to pivotal clinical development.
- Substitution of Titanium Dioxide in Oral Dosage Forms: In July 2022, the European Medicines Agency released technical recommendations addressing the removal or replacement of titanium dioxide in oral pharmaceutical products. This regulatory development has stimulated research into compliant alternative opacifying and whitening agents, such as calcium carbonate, aligned with EU safety standards.
Key Use Cases
- Abuse-Deterrent Opioid Formulations: Two novel excipient submissions aimed at enhancing viscosity and gel strength in opioid tablets were selected during the initial phase of the FDA’s PRIME initiative. These materials are being assessed for their ability to deter tampering and limit rapid drug release, thereby contributing to abuse-deterrent formulation strategies.
- Generic Reformulation through Suitability Petitions: In FY 2024, the FDA achieved its target of reviewing up to 50 excipient suitability petitions within six months. This pathway allows generic manufacturers to substitute approved excipients such as microcrystalline cellulose with functionally comparable alternatives, facilitating market entry without the need for full new drug applications.
- Application of Co-Processed Excipients in Solid Dosage Forms: Immediate-release tablet manufacturers are increasingly integrating CoPEs categorized by the EMA into Risk Categories I (low), II (moderate), and III (high), based on compositional complexity. By consolidating multiple functional properties such as filler and binder into one to three materials, formulation complexity and production steps are reduced.
- IND-Stage Formulation Optimization: Sponsors engaging with the FDA during the IND stage have reported improved formulation outcomes, including enhanced bioavailability and optimized safety assessments. In several development programs, excipient-related consultations occurred within approximately six weeks of IND submission, supporting accelerated initiation of first-in-human studies.
- Titanium Dioxide-Free Formulations: By mid-2022, more than 30 marketing authorization holders across the EU had submitted regulatory variations to replace titanium dioxide in solid oral products. Alternatives such as calcium carbonate have been adopted to align with updated regulatory guidance and evolving safety considerations.
Frequently Asked Questions on Pharma Excipients
- What functions do pharma excipients perform in drug formulations?
Pharma excipients perform critical roles such as fillers, binders, disintegrants, lubricants, coatings, and stabilizers. These functions improve drug solubility, extend shelf life, control release profiles, and enhance manufacturability, contributing significantly to therapeutic efficiency and regulatory compliance. - What are the major types of pharma excipients?
Major types of pharma excipients include organic chemicals, inorganic materials, polymers, and co-processed excipients. They are further categorized as fillers, disintegrants, emulsifiers, preservatives, and coloring agents, depending on their functional properties and application in pharmaceutical formulations. - How is the pharma excipients market segmented?
The pharma excipients market is segmented by product type, functionality, formulation, and geography. By formulation, it includes oral, topical, parenteral, and advanced drug delivery systems. Regional segmentation typically covers North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. - What factors are driving the growth of the pharma excipients market?
Market growth is driven by rising pharmaceutical production, increasing demand for generic drugs, expansion of biologics, and advancements in drug delivery technologies. Growing aging populations and chronic disease prevalence further contribute to sustained demand for high-quality excipients globally. - Which regions dominate the pharma excipients market?
North America and Europe dominate due to established pharmaceutical industries and strong regulatory frameworks. However, Asia-Pacific is witnessing the fastest growth, supported by expanding generic manufacturing, cost advantages, and increasing investments in pharmaceutical infrastructure. - How do regulatory standards impact pharma excipients?
Regulatory standards significantly influence excipient manufacturing, documentation, and quality control. Compliance with pharmacopeial standards and guidelines issued by authorities such as the FDA and EMA is essential to ensure product safety, traceability, and consistent performance. - What trends are shaping the pharma excipients market?
Emerging trends include the development of multifunctional and co-processed excipients, growth in biologics and biosimilars, adoption of quality-by-design practices, and increasing preference for plant-based and sustainable excipient materials to meet evolving industry requirements.
Conclusion
The global pharmaceutical excipients market is positioned for sustained expansion, driven by increasing drug production, rising chronic disease prevalence, and continuous innovation in formulation technologies. Market valuation is projected to reach US$ 18.8 billion by 2033, supported by a CAGR of 7.3%. Strong regulatory frameworks, particularly in Europe and North America, are reinforcing product quality and safety standards, while Asia-Pacific is emerging as a high-growth region.
Growing adoption of multifunctional, co-processed, and modified-release excipients reflects evolving formulation needs. Overall, advancements in biologics, generics, and controlled-delivery systems are expected to maintain steady long-term market momentum.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



