Table of Contents
Overview
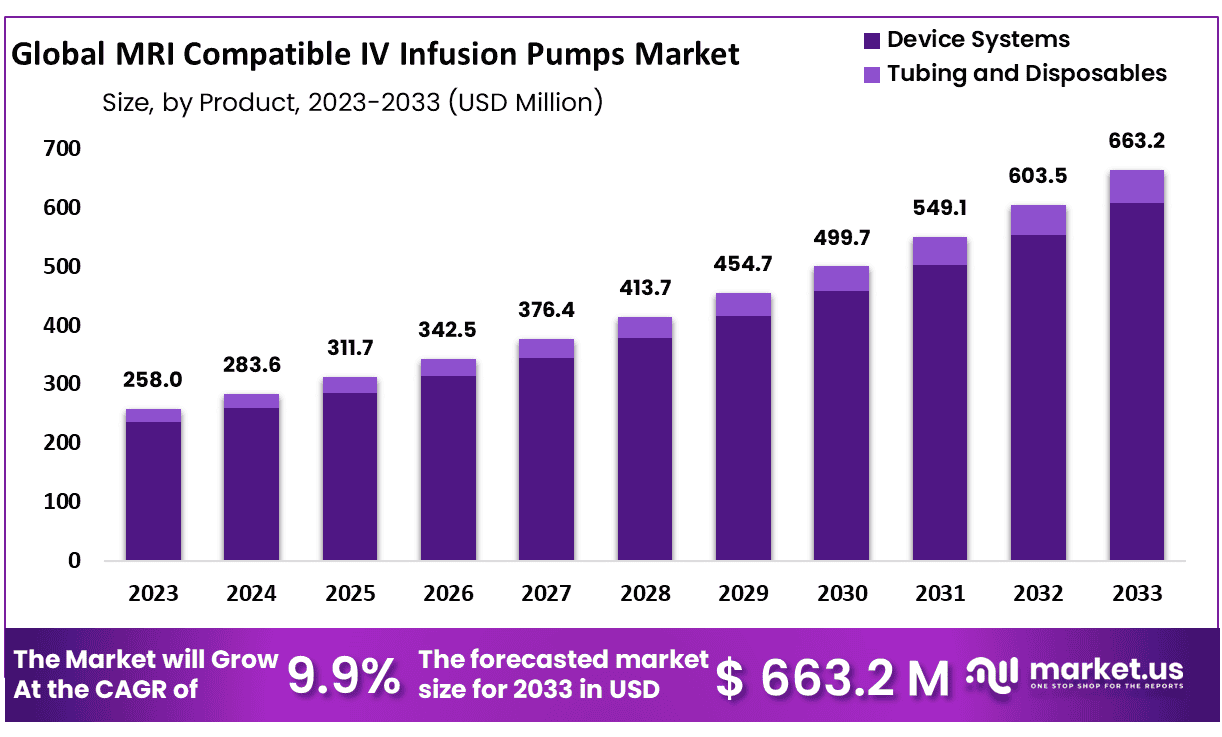
New York, NY – Jan 21, 2026 – The Global MRI Compatible IV Infusion Pumps Market size is expected to be worth around USD 663.2 Million by 2033 from USD 258.0 Million in 2023, growing at a CAGR of 9.9% during the forecast period from 2024 to 2033.
The adoption of MRI compatible IV infusion pumps is increasing steadily, driven by the growing demand for safer and more efficient drug delivery systems in magnetic resonance imaging environments. These infusion pumps are specifically designed to operate accurately within high magnetic fields, minimizing risks associated with conventional infusion devices during MRI procedures.
MRI compatible IV infusion pumps are manufactured using non-magnetic materials and advanced shielding technologies. This design ensures uninterrupted medication delivery while preventing image distortion and equipment interference. As a result, patient safety is significantly improved, particularly for critically ill patients who require continuous infusion during diagnostic imaging.
The growth of the market can be attributed to the rising number of MRI procedures worldwide, increasing prevalence of chronic diseases, and expanding use of MRI in emergency and intensive care settings. Healthcare facilities are increasingly prioritizing equipment that supports seamless clinical workflows without compromising diagnostic accuracy.
Hospitals and diagnostic centers are adopting these pumps to comply with stringent safety standards and regulatory requirements. In addition, technological advancements such as wireless monitoring, programmable flow rates, and enhanced alarm systems are contributing to improved clinical efficiency and reduced manual intervention.
Manufacturers are focusing on product innovation, ergonomic design, and compatibility with a wide range of infusion therapies. Strategic collaborations with healthcare providers are also supporting broader adoption across developed and emerging markets.
Overall, MRI compatible IV infusion pumps are positioned as a critical component in modern imaging suites, supporting safe patient management and high-quality diagnostic outcomes. Continued investment in healthcare infrastructure is expected to further support market expansion over the coming years.
Key Takeaways
- Market Size: The MRI Compatible IV Infusion Pumps market is projected to reach approximately USD 663.2 million by 2033, rising from USD 258.0 million in 2023.
- Market Growth: The market is anticipated to expand at a compound annual growth rate (CAGR) of 9.9% over the forecast period from 2024 to 2033.
- Product Analysis: The device systems segment dominates the market, accounting for a substantial 91.5% share, reflecting strong demand for integrated and reliable infusion solutions.
- End-Use Analysis: Hospitals represent the leading end-use segment, capturing 64.22% of the total market share in 2023, driven by high MRI procedure volumes and critical care needs.
- Regional Analysis: North America leads the global market with an estimated 52.3% market share, generating approximately USD 134.9 million in revenue.
- Technological Advancements: Significant technological progress has led to the development of advanced MRI-compatible infusion pumps featuring enhanced safety mechanisms and precise drug delivery during imaging procedures.
- Regulatory Compliance: Market participants are required to comply with strict regulatory frameworks established by the U.S. Food and Drug Administration (FDA) and other international regulatory authorities to ensure product approval and sustained market presence.
- MRI System Integration: Manufacturers are increasingly focusing on seamless integration of infusion pumps with MRI systems to support streamlined clinical workflows and improved patient care delivery.
Regional Analysis
On a regional basis, the global MRI-compatible IV infusion pumps market is segmented into North America, Europe, Asia-Pacific, South America, and the Middle East & Africa. North America dominates the market, accounting for approximately 52.3% of the total market share and generating around USD 134.9 million in revenue. Among all regions, North America is expected to continue holding the largest revenue share in the global MRI-compatible IV infusion pumps market throughout the forecast period.
Emerging Trends
- Technological Advancements: Product development efforts are increasingly focused on infusion pumps that can operate reliably within high-strength magnetic resonance environments. For example, the MRidium™ 3850 MRI Infusion Pump System is engineered to function safely in magnetic fields of up to 10,000 gauss, ensuring consistent performance during MRI procedures.
- Improved Safety Mechanisms: Contemporary MRI-compatible infusion pumps are equipped with advanced safety features designed to mitigate risks associated with strong magnetic fields. These mechanisms support enhanced patient and clinician safety during imaging procedures.
- Regulatory Clearances and Standards Compliance: Manufacturers are prioritizing regulatory approvals to validate device safety and effectiveness. The FDA clearance of the MRidium™ 3860 MRI Infusion Pump/Monitoring System reflects adherence to rigorous regulatory and safety requirements.
Key Use Cases
- Critical Care Applications: Patients in intensive care settings often require continuous infusion of critical medications, including sedatives and vasopressors. MRI-compatible infusion pumps enable uninterrupted drug delivery during MRI examinations, supporting patient stability.
- Pediatric Imaging: Pediatric patients frequently require sedation to remain immobile during MRI scans. MRI-compatible infusion pumps allow for the safe and controlled administration of sedatives, facilitating accurate imaging while maintaining safety standards.
- Oncology Care: Oncology patients undergoing chemotherapy may need periodic MRI scans for disease monitoring. MRI-compatible infusion pumps support the continuation of chemotherapy infusions during imaging, minimizing treatment delays and enhancing patient comfort.
- Emergency Care Settings: In emergency departments, patients may require urgent MRI diagnostics while receiving life-sustaining intravenous therapies. MRI-compatible infusion pumps allow concurrent imaging and treatment, enabling faster clinical decision-making and intervention.
Frequently Asked Questions on MRI Compatible IV Infusion Pumps
- How do MRI compatible infusion pumps ensure patient safety?
Patient safety is ensured through non-ferrous construction, controlled electromagnetic emissions, extended tubing systems, and rigorous testing under high magnetic field strengths, allowing accurate drug delivery without interference with MRI operations. - In which clinical applications are MRI compatible IV pumps commonly used?
These pumps are widely used in neuroimaging, cardiac MRI, pediatric imaging, and critical care diagnostics, where continuous infusion of contrast agents, anesthetics, or life-sustaining medications is required during prolonged MRI scans. - What magnetic field strengths can MRI compatible IV pumps support?
Most MRI compatible IV infusion pumps are designed to operate safely within 1.5T and 3T MRI systems, with select advanced models supporting higher field strengths based on manufacturer specifications and regulatory compliance. - What factors are driving the growth of the MRI compatible IV infusion pumps market?
Market growth is primarily driven by rising MRI procedure volumes, increasing demand for patient safety, expansion of advanced diagnostic imaging, and stringent regulatory standards requiring MRI-safe medical equipment in healthcare facilities. - How is technological innovation influencing this market?
Technological advancements have improved pump accuracy, wireless monitoring, battery efficiency, and MRI-conditional labeling, enhancing clinical usability and encouraging hospitals to replace conventional systems with MRI-compatible infusion technologies. - Which end users dominate the MRI compatible IV infusion pumps market?
Hospitals, diagnostic imaging centers, and specialty clinics dominate market demand, as these facilities conduct high MRI scan volumes and require reliable infusion solutions to support complex diagnostic and interventional imaging procedures. - What regional trends are observed in the global market?
North America leads due to advanced healthcare infrastructure and early technology adoption, while Asia-Pacific shows the fastest growth, supported by expanding imaging capacity, rising healthcare investments, and increasing awareness of MRI safety standards.
Conclusion
The MRI compatible IV infusion pumps market is experiencing steady expansion, supported by rising MRI procedure volumes, increasing patient safety requirements, and growing use of advanced imaging in critical care settings. The adoption of non-magnetic materials, enhanced shielding, and intelligent safety features has strengthened clinical confidence in these systems.
Hospitals remain the primary end users, driven by regulatory compliance and workflow efficiency needs. North America continues to dominate due to strong healthcare infrastructure, while emerging regions present high growth potential. Overall, continuous technological innovation and sustained healthcare investment are expected to reinforce long-term market growth and clinical relevance.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



