Table of Contents
Overview
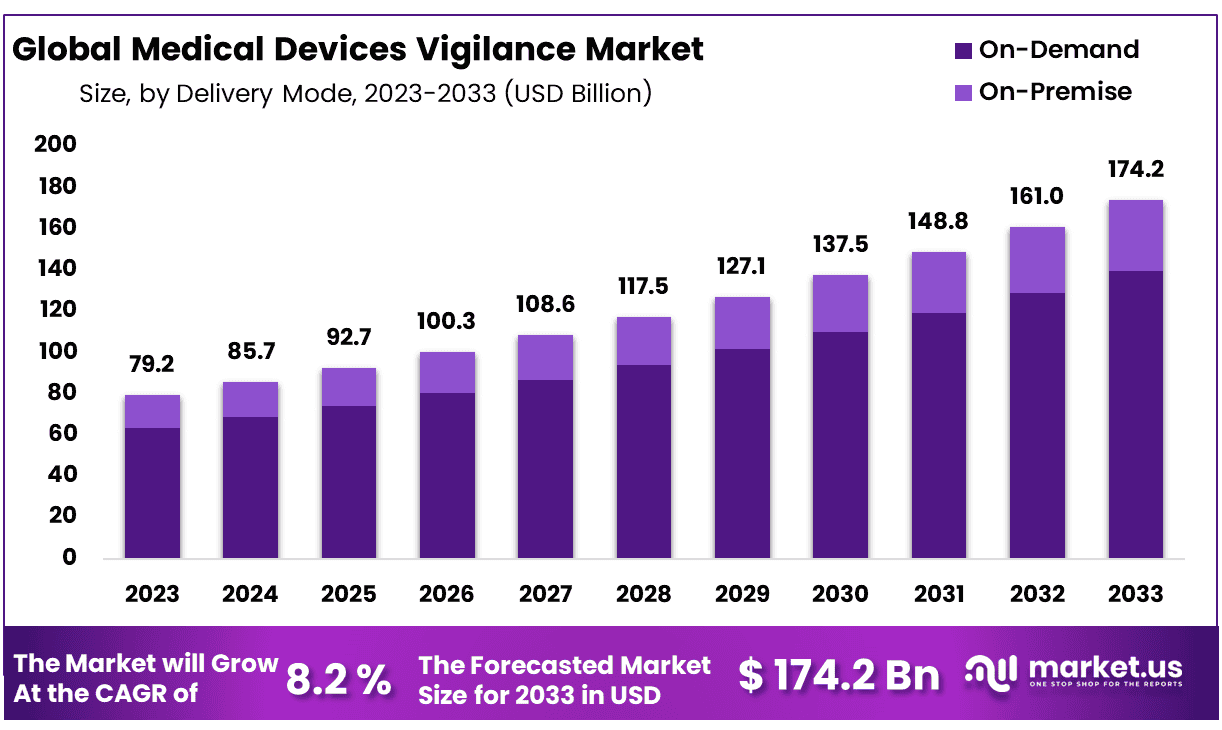
New York, NY – Jan 28, 2026 – The Global Medical Devices Vigilance Market size is expected to be worth around USD 174.2 Billion by 2033 from USD 79.2 Billion in 2023, growing at a CAGR of 8.2% during the forecast period from 2024 to 2033.
A Medical Devices Vigilance Basic Formation program has been introduced to strengthen awareness, compliance, and best practices in post-market surveillance of medical devices. The program is designed to provide foundational knowledge on vigilance systems, regulatory requirements, and reporting obligations, supporting healthcare professionals, manufacturers, and regulatory stakeholders in ensuring patient safety.
The formation focuses on the core principles of medical device vigilance, including identification and assessment of incidents, timely reporting to competent authorities, and implementation of corrective and preventive actions. Emphasis is placed on understanding the roles and responsibilities of manufacturers, distributors, healthcare institutions, and authorized representatives within the vigilance framework. The growth in the use of complex medical devices has increased the importance of structured vigilance systems, which are essential for early risk detection and mitigation.
Participants will gain a clear overview of international and regional regulatory expectations, including documentation practices, signal detection, and trend reporting. Practical case examples are incorporated to illustrate common vigilance scenarios and regulatory responses, ensuring that theoretical concepts are linked to real-world applications. The training also highlights the importance of data quality, traceability, and effective communication with regulatory bodies.
This basic formation program is positioned as an essential step for organizations seeking to build or reinforce compliant vigilance processes. By enhancing competency in medical devices vigilance, the initiative contributes to improved patient safety outcomes, regulatory compliance, and continuous improvement across the medical technology sector.
Key Takeaways
- Market Size: The Medical Devices Vigilance market is projected to reach approximately USD 174.2 billion by 2033, rising from USD 79.2 billion in 2023.
- Market Growth: The market is anticipated to expand at a compound annual growth rate (CAGR) of 8.2% over the forecast period from 2024 to 2033.
- Delivery Mode Analysis: On-demand delivery emerges as the leading segment, accounting for nearly 80% of the overall market share.
- Application Analysis: The diagnostics segment represents a significant portion of the market, contributing around 34% of total revenue.
- End-Use Analysis: Clinical Research Organizations (CROs) dominate the end-use landscape, holding approximately 40% of the market share.
- Regional Analysis: North America leads the global market with a 33% share, generating an estimated revenue of USD 26.1 billion.
Regional Analysis
North America accounts for a substantial share of the market, representing approximately 33% of total revenue and generating around USD 26.1 billion. This strong position can be attributed to the rising number of reported adverse events, which has accelerated the adoption of medical device vigilance systems across the region.
In parallel, the Asia–Pacific region is expected to register the fastest growth rate over the forecast period from 2024 to 2033. This expansion is supported by the presence of a large and heterogeneous patient base, along with the increasing inclination toward outsourcing clinical research activities to countries within the region.
The country-level analysis included in the medical devices vigilance market report provides in-depth insights into nation-specific factors shaping market performance. It also highlights domestic regulatory developments that are anticipated to influence both current market dynamics and long-term growth trajectories.
Emerging Trends
- Integration of Artificial Intelligence (AI): The U.S. Food and Drug Administration has issued new guidelines enabling faster approvals for AI-enabled medical devices. These guidelines permit iterative updates without full resubmission, supporting rapid performance improvements and adaptive functionality.
- Advancements in Medical Robotics: Medical robotics development is accelerating, with companies such as Mendaera integrating robotics with ultrasound guidance to enhance procedural precision. In 2023, venture capital funding in medical robotics reached USD 767 million, indicating strong investor confidence.
- Impact of Trade Policies on Supply Chains: Trade tariffs on imports from China, Mexico, and Canada have disrupted medical device supply chains, increasing production costs. In response, some manufacturers are exploring re-shoring strategies, despite operational and financial challenges.
Use Cases
- Smartwatch Monitoring for Atrial Fibrillation (AFib): Consumer smartwatches from Apple and Samsung enable early detection of irregular heart rhythms associated with AFib. However, these devices currently contribute only marginally to overall new AFib diagnosis rates.
- Regulatory Actions on Device Safety: The FDA designated the recall of Boston Scientific’s POLARx Cryoablation Balloon Catheters as a Class I recall following reports of serious injuries and fatalities, emphasizing the critical role of proactive post-market vigilance.
- International Trade and Market Access: The European Commission has identified discriminatory procurement practices affecting EU medical device suppliers in China. Potential reciprocal measures may follow, underscoring the importance of vigilance in maintaining regulatory compliance and equitable global market access.
Frequently Asked Questions on Medical Devices Vigilance
- Why is medical devices vigilance important for healthcare systems?
Medical devices vigilance is important because it helps identify device-related risks early, reduces patient harm, supports evidence-based regulatory decisions, and enhances overall healthcare quality by ensuring continuous post-market safety surveillance. - What types of incidents are reported under medical devices vigilance?
Reported incidents typically include device malfunctions, serious injuries, near-miss events, and deaths linked to medical device use, along with trends that indicate recurring safety issues requiring investigation or regulatory intervention. - Who is responsible for reporting vigilance data?
Manufacturers, healthcare professionals, importers, distributors, and sometimes patients are responsible for reporting vigilance data, depending on regional regulations, ensuring comprehensive post-market surveillance and regulatory transparency. - What factors are driving growth in the medical devices vigilance market?
Market growth is driven by increasing medical device approvals, stricter regulatory requirements, rising patient safety concerns, and growing adoption of digital vigilance platforms to automate reporting and compliance workflows. - How does regulation influence the medical devices vigilance market?
Regulatory frameworks mandate structured vigilance systems, increasing demand for compliant software and services, while frequent updates to global regulations encourage continuous investment in advanced, scalable vigilance solutions. - What are the key end users in the medical devices vigilance market?
Key end users include medical device manufacturers, contract research organizations, regulatory consultants, and healthcare institutions that require robust systems to manage post-market safety data and regulatory reporting obligations.
Conclusion
In conclusion, the medical devices vigilance landscape continues to evolve in response to increasing device complexity, stringent regulatory requirements, and growing emphasis on patient safety. The introduction of structured basic formation programs strengthens stakeholder awareness, enhances compliance, and supports effective post-market surveillance practices.
Market expansion is driven by technological advancements, particularly in artificial intelligence and robotics, alongside rising adverse event reporting. Regional growth patterns highlight North America’s market leadership and Asia–Pacific’s rapid expansion potential.
Collectively, these dynamics underscore the critical role of robust vigilance systems in ensuring regulatory alignment, mitigating risks, and fostering sustainable growth across the global medical technology sector.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



