Table of Contents
Overview
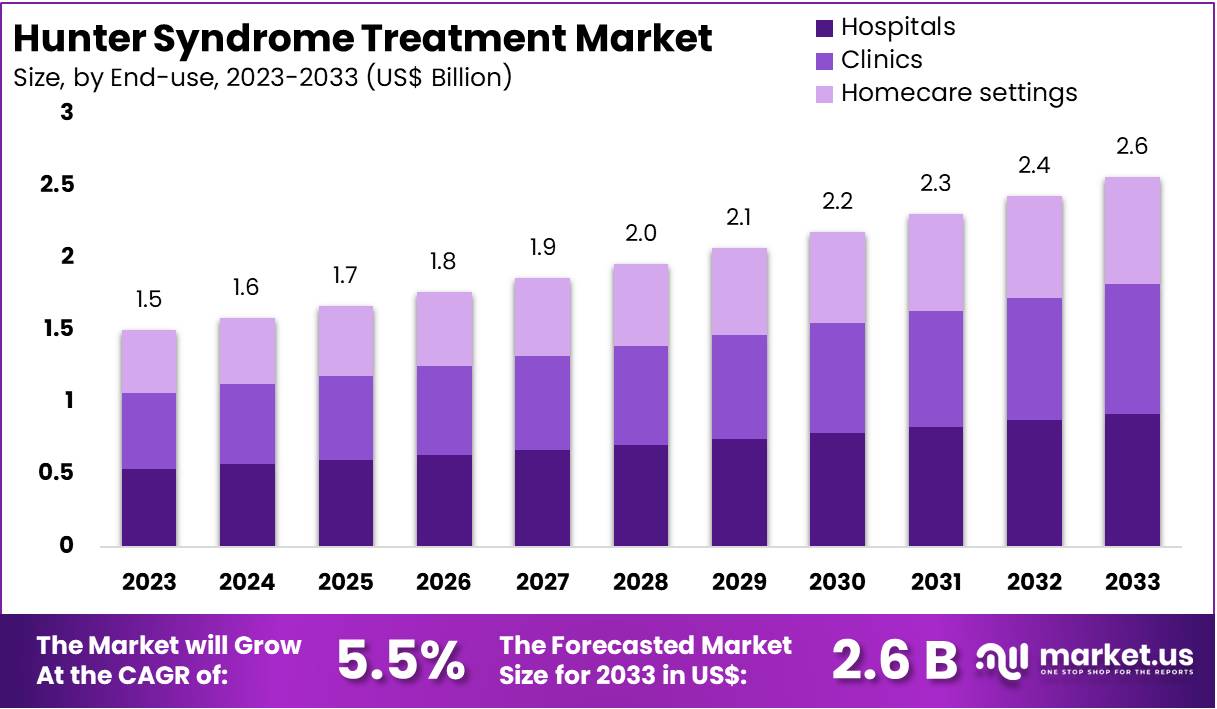
New York, NY – Feb 18, 2026 – The Global Hunter Syndrome Treatment Market size is expected to be worth around US$ 2.6 Billion by 2033, from US$ 1.5 Billion in 2023, growing at a CAGR of 5.5% during the forecast period from 2024 to 2033. North America maintained a leading position in the market, accounting for over 38% of the share, with a market value of approximately US$ 0.5 billion.
A structured strategic framework has been introduced to advance innovation in Hunter syndrome treatment, addressing a critical unmet need within the rare disease therapeutic landscape. Hunter syndrome, also known as Mucopolysaccharidosis Type II (MPS II), is a rare, inherited lysosomal storage disorder characterized by deficient iduronate-2-sulfatase enzyme activity, leading to progressive multi-organ complications.
The newly outlined development initiative is focused on enhancing enzyme replacement therapies (ERT), advancing gene therapy platforms, and strengthening supportive care approaches to improve long-term clinical outcomes. Emphasis is being placed on next-generation treatment modalities designed to address neurological manifestations, which remain inadequately managed through conventional therapies.
Strategic collaborations with biotechnology innovators, research institutions, and regulatory authorities are being reinforced to accelerate clinical development and streamline approval pathways. Advanced technologies, including gene-editing tools and precision delivery systems, are being evaluated to improve therapeutic efficacy and reduce disease burden.
The global Hunter syndrome treatment market is anticipated to witness steady growth, supported by increasing disease awareness, expanded newborn screening programs, and rising investment in orphan drug development. Regulatory incentives for rare disease therapies continue to encourage pipeline expansion and commercialization efforts.
This foundational advancement reflects a long-term commitment to improving patient quality of life, expanding access to innovative therapies, and strengthening the rare disease treatment ecosystem through sustained research and strategic investment initiatives.
Key Takeaways
- The global Hunter Syndrome Treatment Market is anticipated to attain a valuation of approximately US$ 2.6 billion by 2033, increasing from US$ 1.5 billion recorded in 2023.
- The market is forecast to register a compound annual growth rate (CAGR) of 5.5% throughout the period from 2024 to 2033.
- In 2023, Enzyme Replacement Therapy (ERT) emerged as the dominant treatment category, capturing more than 76% of the overall market share.
- Hospitals represented the leading end-use segment in 2023, contributing over 76% of total revenue within the Hunter Syndrome Treatment Market.
- North America secured the largest regional share in 2023, accounting for more than 38% of the global market revenue.
Regional Analysis
In 2023, North America maintained a leading position in the Hunter Syndrome Treatment Market, accounting for over 38% of global revenue and reaching a market value of approximately US$ 0.5 billion. This dominance has been supported by high diagnosis rates, advanced healthcare infrastructure, and increased awareness of genetic disorders, enabling early detection and timely therapeutic intervention.
The region is characterized by the strong presence of major pharmaceutical companies that continue to invest substantially in research and development. Ongoing innovation has resulted in the introduction of advanced and targeted therapies designed to address the complex clinical manifestations of Hunter Syndrome, ensuring consistent availability of effective treatment options.
Government support has further strengthened the regional market through favorable reimbursement frameworks, rare disease subsidies, and specialized healthcare programs. In addition, strategic collaborations between research institutions and healthcare providers have facilitated clinical advancements and improved treatment accessibility, reinforcing North America’s leadership in therapeutic innovation and patient care standards.
Emerging Trends
- One-Time Gene Therapy Modalities: Single-administration gene therapies based on adeno-associated virus vectors are being evaluated to deliver a functional IDS gene. Clinical programs such as RGX-121 are assessing whether durable enzyme restoration can reduce reliance on lifelong weekly infusions and lower treatment burden.
- Intrathecal Enzyme Replacement for Neurological Involvement: Intrathecal enzyme replacement therapy has been explored to overcome the blood–brain barrier and target central nervous system manifestations. Idursulfase-IT received orphan drug designation from the U.S. FDA in 2009, reflecting a strategic shift toward addressing neurocognitive complications.
- Substrate Reduction Therapy Development: Substrate reduction therapy focuses on small molecules designed to inhibit glycosaminoglycan synthesis. Early-stage research indicates that this approach may reduce intracellular substrate accumulation and potentially complement enzyme replacement therapy, with the added advantage of future oral administration.
- Hematopoietic Stem Cell and Gene-Modified Therapies: Therapeutic strategies combining hematopoietic stem cell transplantation with gene-editing technologies are under investigation. These approaches aim to introduce corrected genetic material into patients’ own cells, enabling sustained systemic enzyme production and improved tissue-level distribution.
- Fusion Protein and Transport-Enhanced Platforms: Innovative fusion protein therapies, including Tividenofusp Alfa (DNL310), are being tested to enhance intracellular enzyme delivery. These engineered constructs incorporate transport domains to improve tissue penetration, potentially enhancing therapeutic effectiveness while reducing infusion frequency.
Use Cases
- Weekly Idursulfase Administration in Pediatric Patients: In a 53-week clinical safety evaluation, young pediatric patients received weekly intravenous idursulfase at 0.5 mg/kg. The therapy demonstrated an acceptable safety profile, with limited infusion-related reactions such as mild rash and occasional nausea.
- Functional and Cardiopulmonary Improvements in Adults: Adult patients undergoing regular idursulfase therapy demonstrated measurable functional benefits, including increased six-minute walk test distance and improved forced vital capacity. Cardiac outcomes were also favorable, with reductions observed in left ventricular mass index following sustained treatment.
- Clinical Outcomes of Idursulfase Beta: A 52-week controlled study involving male participants indicated that idursulfase beta achieved superior outcomes compared to placebo. Significant improvements were recorded in walking distance, urinary glycosaminoglycan reduction, and reductions in liver and spleen volumes, supporting its therapeutic efficacy.
Frequently Asked Questions on Hunter Syndrome Treatment
- What is enzyme replacement therapy for Hunter Syndrome?
Enzyme replacement therapy involves intravenous administration of synthetic iduronate-2-sulfatase to compensate for the missing enzyme. This therapy helps reduce glycosaminoglycan accumulation, improves physical symptoms, and enhances quality of life in affected patients. - Are there any advanced therapies under development for Hunter Syndrome?
Advanced therapies, including gene therapy and substrate reduction therapy, are under clinical investigation. These approaches aim to provide long-term therapeutic benefits by addressing the underlying genetic defect, potentially offering improved outcomes compared to conventional treatments. - What factors are driving the Hunter Syndrome Treatment Market growth?
Growth of the Hunter Syndrome Treatment Market is driven by increasing awareness of rare diseases, advancements in biotechnology, rising healthcare expenditure, and supportive regulatory incentives such as orphan drug designations across developed and emerging economies. - Which regions dominate the Hunter Syndrome Treatment Market?
North America currently dominates the market due to strong healthcare infrastructure, early diagnosis rates, and favorable reimbursement policies. Europe follows closely, while Asia-Pacific is expected to witness steady growth supported by improving healthcare investments and awareness programs. - Who are the key players in the Hunter Syndrome Treatment Market?
Major companies operating in this market include Takeda Pharmaceutical Company Limited, Denali Therapeutics Inc., and other biotechnology firms focusing on rare genetic disorders. Strategic collaborations and pipeline expansions remain key competitive strategies within the industry. - What is the future outlook of the Hunter Syndrome Treatment Market?
The market outlook remains cautiously optimistic, supported by innovation in gene therapies and expanding orphan drug incentives. Continued research investment and improved diagnostic screening are expected to enhance treatment adoption and drive moderate long-term revenue growth.
Conclusion
The Hunter Syndrome Treatment Market is positioned for stable expansion, supported by continued innovation across enzyme replacement, gene therapy, and advanced biologic platforms. Market growth is projected to reach approximately US$ 2.6 billion by 2033, driven by rising awareness, regulatory incentives, and expanding orphan drug pipelines.
North America remains the leading regional contributor, while emerging therapeutic modalities are addressing long-standing neurological and systemic challenges. Strategic collaborations and sustained research investments are strengthening the rare disease ecosystem. Collectively, these advancements are expected to improve long-term clinical outcomes, expand treatment accessibility, and reinforce the market’s sustainable development trajectory.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



