Table of Contents
Overview
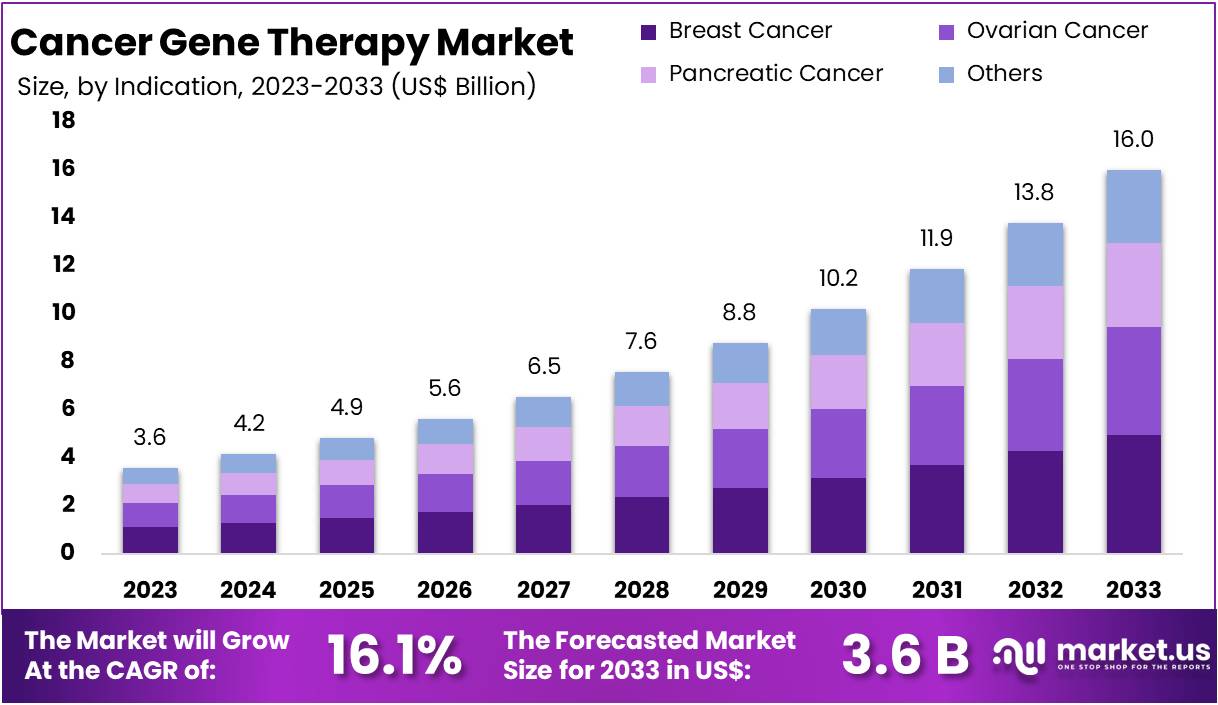
New York, NY – Feb 13, 2026 – The Global Cancer Gene Therapy Market size is expected to be worth around US$ 16 Billion by 2033, from US$ 3.6 Billion in 2023, growing at a CAGR of 16.1% during the forecast period from 2024 to 2033. North America emerged as the dominant region, securing over 36.5% of the market share with a valuation of US$ 1.3 billion for the year.
Cancer gene therapy represents a significant advancement in modern oncology, focusing on modifying or replacing defective genes responsible for tumor development and progression. This innovative therapeutic approach is designed to target cancer at the molecular level, offering a more precise and personalized alternative to conventional treatments such as chemotherapy and radiation therapy.
The basic foundation of cancer gene therapy involves the introduction, removal, or alteration of genetic material within a patient’s cells. Functional genes are delivered into cancer cells using safe viral or non-viral vectors, enabling the correction of abnormal genetic activity. In some approaches, genes are introduced to stimulate the immune system to recognize and destroy malignant cells more effectively. Other strategies aim to deactivate oncogenes or restore tumor suppressor genes that regulate normal cell growth.
Clinical trials have demonstrated promising outcomes across multiple cancer types, including leukemia, lymphoma, and solid tumors. Advancements in gene-editing technologies, such as CRISPR-based platforms, have further enhanced precision and therapeutic potential.
The growth of cancer gene therapy research can be attributed to increasing cancer incidence worldwide and rising investment in advanced biotechnologies. As regulatory frameworks evolve and clinical evidence expands, gene therapy is positioned to play a transformative role in oncology care.
This approach reflects a shift toward targeted, durable, and potentially curative cancer treatments, marking a new era in precision medicine.
Key Takeaways
- By 2033, the Global Cancer Gene Therapy Market is anticipated to attain an estimated valuation of nearly US$ 16 billion, expanding at a compound annual growth rate (CAGR) of 16.1% during the forecast period.
- In 2023, Gene Induced Immunotherapy emerged as the dominant therapy type within the Cancer Gene Therapy Market, accounting for 38.22% of the overall market share.
- Within the indication segment, Gene Induced Immunotherapy also maintained a leading position in 2023, representing 31.51% of the total market share.
- Biopharmaceutical companies constituted the primary end-user segment in 2023, contributing 48.62% to the overall Cancer Gene Therapy Market revenue.
- North America led the regional landscape in 2023, holding a 36.5% market share, with the regional market valued at approximately US$ 1.3 billion.
Regional Analysis
In 2023, North America maintained a leading position in the Cancer Gene Therapy Market, accounting for over 36.5% of total revenue and reaching a market value of approximately US$ 1.3 billion. Market dominance was supported by the high prevalence of cancer, which has sustained strong demand for advanced therapeutic solutions. The presence of a well-developed healthcare infrastructure and continuous investment in biotechnology and medical research further strengthened regional growth.
The region benefits from advanced technological capabilities and the concentration of major biotechnology firms and research institutions engaged in genetic and oncology innovation. Favorable government initiatives, along with substantial public and private funding, have accelerated research and commercialization activities.
In addition, a structured regulatory environment has enabled relatively faster approvals of gene-based therapies, facilitating timely market entry. Higher patient awareness and participation in clinical trials have also supported efficient study enrollment and data generation. Collectively, these factors have reinforced North America’s leadership in the global market.
Emerging Trends in Cancer Gene Therapy
- mRNA-Based Delivery Systems: Messenger RNA platforms are increasingly utilized for delivering therapeutic genetic material in oncology applications. These systems allow flexible payload design and transient gene expression, while minimizing the risk of insertional mutagenesis commonly associated with DNA-based vectors, thereby improving safety profiles.
- Nanoparticle Carriers for Targeted Delivery: Lipid- and polymer-based nanoparticles are being widely adopted to enhance gene delivery efficiency. These carriers protect genetic payloads from degradation in circulation and enable targeted tumor localization, resulting in improved cellular uptake, enhanced therapeutic precision, and reduced off-target toxicity.
- Advanced Viral Vector Engineering: Next-generation viral vectors, including engineered adenoviruses and lentiviruses, are being optimized for improved transduction efficiency and biosafety. Structural modifications are incorporated to limit replication in healthy tissues, thereby enhancing tumor specificity and reducing adverse systemic effects.
- CRISPR/Cas9 Gene Editing for Personalized Immunotherapy: CRISPR/Cas9 technology is being applied to precisely modify patient-derived immune cells for individualized cancer treatment. Gene edits such as PD-1 knockout or insertion of tumor-specific receptors enhance immune activity, improve therapeutic targeting, and support strategies to overcome tumor resistance mechanisms.
Key Use Cases in Cancer Gene Therapy
- CAR T-Cell Therapy for Hematological Malignancies: CAR T-cell therapy involves harvesting patient T cells, genetically modifying them to express chimeric antigen receptors, expanding them ex vivo, and reinfusing them into the patient. Since 2017, six therapies have received regulatory approval, with manufacturing timelines typically ranging from three to five weeks.
- T-Cell Receptor (TCR) Therapy for Solid Tumors: Afamitresgene autoleucel has been approved for the treatment of metastatic synovial sarcoma. In a clinical study involving 44 patients, tumor reduction was observed in 43% of participants, with a median response duration of approximately six months.
- GD2-Targeted CAR T Therapy for Diffuse Midline Gliomas: GD2-directed CAR T-cell therapy has demonstrated promising outcomes in early trials involving pediatric and young adult patients. In a small cohort of 11 individuals, intracranial infusions resulted in measurable tumor reduction, with several patients surviving beyond two years post-treatment.
- CRISPR-Engineered T Cells in Early Clinical Development: A first-in-human Phase I clinical study utilized CRISPR/Cas9 to remove endogenous T-cell receptor genes and PD-1 expression in patient T cells. Three individuals with refractory cancers tolerated the therapy, confirming feasibility and an acceptable preliminary safety profile.
Frequently Asked Questions on Cancer Gene Therapy
- How does cancer gene therapy work?
Cancer gene therapy works by delivering therapeutic genes into cancer cells using vectors such as modified viruses or non-viral delivery systems. These genes can replace mutated genes, trigger cell death in tumors, or enhance the immune system’s ability to recognize and destroy malignant cells. - What are the main types of cancer gene therapy?
The primary types include gene replacement therapy, gene silencing, suicide gene therapy, and immunogene therapy. These approaches focus on restoring normal gene function, blocking oncogene activity, inducing tumor cell apoptosis, or enhancing immune-mediated destruction of cancer cells. - What are the benefits of cancer gene therapy?
Cancer gene therapy offers targeted treatment with the potential to minimize damage to healthy tissues compared to conventional chemotherapy or radiation. It supports precision medicine by addressing genetic mutations at the molecular level, improving treatment efficacy and long-term patient outcomes. - What factors are driving the growth of the cancer gene therapy market?
Market growth is driven by increasing cancer prevalence, rising demand for targeted therapies, and advancements in gene editing technologies such as CRISPR. Growing investments in biotechnology research and supportive regulatory pathways are further accelerating product development and commercialization. - Which regions dominate the cancer gene therapy market?
North America dominates the market due to strong research infrastructure, significant funding, and a high number of clinical trials. Europe follows with increasing regulatory support, while Asia-Pacific is emerging rapidly due to expanding healthcare expenditure and biotechnology investments. - Who are the key players in the cancer gene therapy market?
Key players include leading biotechnology and pharmaceutical companies engaged in advanced gene therapy research and commercialization. These organizations focus on strategic collaborations, mergers, and pipeline expansion to strengthen market position and accelerate innovation in oncology gene therapies. - What is the future outlook for the cancer gene therapy market?
The market outlook remains positive, supported by a strong clinical pipeline and increasing regulatory approvals. Continued innovation in vector design, personalized medicine approaches, and manufacturing scalability is expected to enhance accessibility and drive sustained market expansion over the forecast period.
Conclusion
Cancer gene therapy has emerged as a transformative modality in oncology, driven by advancements in molecular biology, gene-editing technologies, and precision medicine strategies. Strong clinical progress, expanding regulatory approvals, and increasing investment in biotechnology have reinforced its commercial viability. The market is projected to reach approximately US$ 16 billion by 2033, supported by a CAGR of 16.1%.
North America continues to lead due to robust infrastructure and funding. With continued innovation in vector engineering, immunogene therapy, and CRISPR-based platforms, cancer gene therapy is expected to enhance treatment outcomes, improve personalization, and establish long-term therapeutic value across multiple cancer indications.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



