Table of Contents
Overview
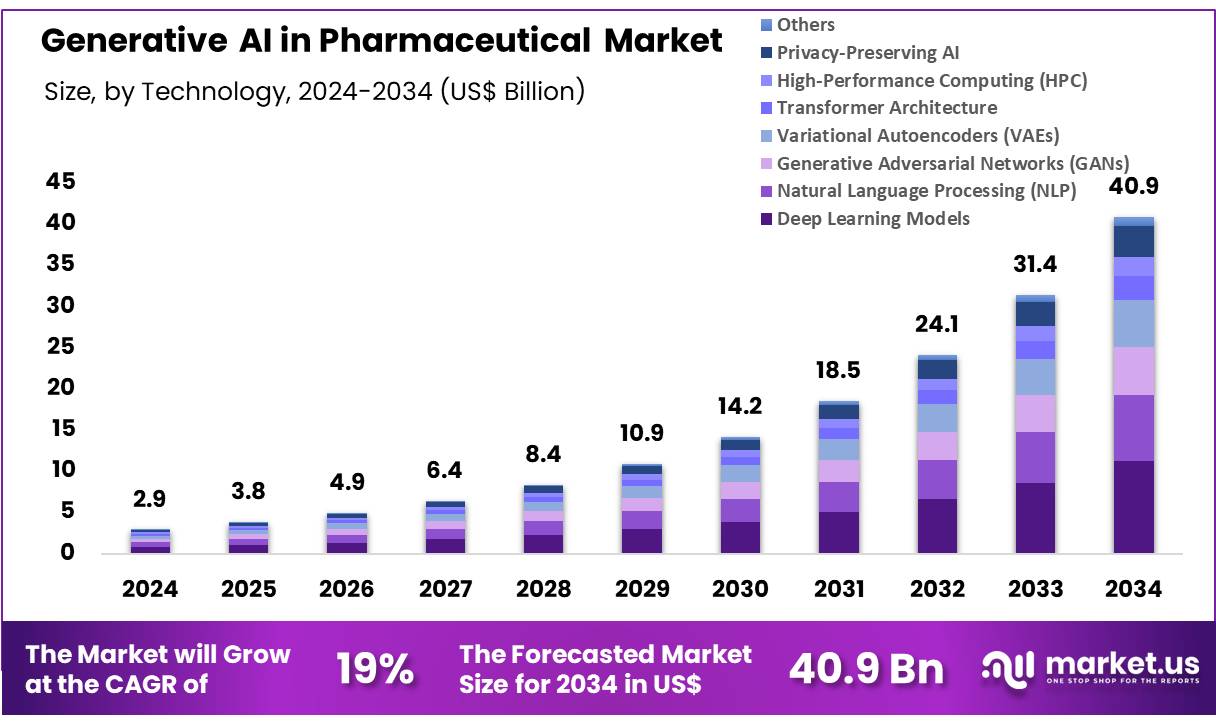
New York, NY – Nov 06, 2025 – Global Generative AI in Pharmaceutical Market size is expected to be worth around US$ 40.88 Billion by 2034 from US$ 2.92 Billion in 2024, growing at a CAGR of 30.2% during the forecast period 2025 to 2034.
The integration of generative artificial intelligence is being recognized as a significant advancement in the pharmaceutical industry. Its adoption has been driven by the need for accelerated drug discovery, improved R&D efficiency, and enhanced decision-making. The value of the global pharmaceutical AI market has been estimated to exceed USD 3.5 billion in 2024, and steady expansion is anticipated as companies increase investments in advanced computational tools.
The application of generative AI has supported the identification of new molecular structures, reduced early-stage development timelines, and improved prediction accuracy for safety and efficacy profiles. Substantial reductions in screening costs have been reported, as advanced models are capable of analyzing complex datasets at scale. The growth of the market has been attributed to strong demand for precision therapies, rising chronic disease prevalence, and increased digital transformation among pharmaceutical manufacturers.
Strategic collaborations between technology providers and pharmaceutical companies have strengthened the deployment of generative AI platforms. These collaborations have enabled the integration of cloud computing, high-performance analytics, and automated workflows. Regulatory bodies have also initiated frameworks to ensure responsible and transparent use of AI in drug development processes.
Overall, the adoption of generative AI is expected to support higher R&D productivity, expand innovation capacity, and improve patient-centric outcomes. Continued investment and supportive regulatory actions are projected to reinforce its role as a critical technology shaping the future of pharmaceutical development.
Key Takeaways
- In 2024, the market for generative AI in the pharmaceutical sector generated revenue of US$ 2.92 billion. A compound annual growth rate of 30.2% has been projected for the forecast period, and the market value is expected to reach US$ 40.88 billion by 2034.
- The technology landscape has been categorized into Deep Learning Models, Natural Language Processing, Generative Adversarial Networks, Variational Autoencoders, Transformer Architecture, High-Performance Computing, Privacy-Preserving AI, and other supporting technologies. Deep Learning Models accounted for the largest share in 2024, representing 27.4 percent of total revenue.
- Based on method, the market has been segmented into Text Generation, Image Generation, Audio Generation, and other emerging generation techniques. Text Generation remained the leading segment, contributing 39.7% to overall revenue in 2024.
- Regarding application, the market has been divided into Commercial, Research and Development, Drug Discovery, Clinical Development, Operations, and other use cases. Research and Development emerged as the dominant application area, holding 22.4% of the revenue share.
- From a regional perspective, North America led the global market in 2024, accounting for 46.8 percent of total revenue, supported by strong technological adoption, advanced healthcare infrastructure, and significant R&D expenditure in the pharmaceutical industry.
Regional Analysis
North America has been identified as the leading region in the generative AI in pharmaceutical market, accounting for 46.8% of the total share in 2024. Its leadership is supported by the rapid adoption of advanced AI technologies and the presence of a mature pharmaceutical ecosystem.
The United States, in particular, hosts several of the largest pharmaceutical and biotechnology companies, as well as prominent AI research institutions, which collectively contribute to a strong foundation for AI-driven drug discovery and development. Generative AI is being applied extensively in areas such as molecular design, clinical trial optimization, and personalized therapeutics, resulting in enhanced operational efficiency and reduced development timelines.
Significant contributions from major technology companies, including Google, Microsoft, and IBM, have further strengthened the region’s position by providing robust AI infrastructure and advanced analytical tools. The regulatory environment in the United States, supported by agencies such as the FDA, is gradually evolving to accommodate AI-enabled processes, thereby creating favorable conditions for wider integration of generative AI across pharmaceutical workflows.
In September 2024, Deloitte introduced AI Factory as a Service, a scalable suite of generative AI capabilities powered by the NVIDIA AI platform. The offering includes NVIDIA AI Enterprise software, NVIDIA NIM Agent Blueprints, and accelerated computing resources in combination with Oracle’s enterprise AI technologies. This initiative reflects a comprehensive approach that integrates Deloitte’s expertise in data science, model development, and industry insights with state-of-the-art AI infrastructure, reinforcing the region’s strong ecosystem for generative AI adoption.
Emerging Trends
- De Novo Drug Design Expansion: The expansion of de novo drug design has been supported by generative AI, enabling creation of novel molecular structures and broader chemical space exploration. By 2025, over 30 percent of new drugs and materials are expected to be discovered through such techniques.
- Automated Regulatory Review Assistance: Regulatory review processes have been strengthened through AI systems such as the FDA’s “Elsa,” which supports summarization of adverse events and protocol evaluations. Review timelines are expected to shorten, improving overall efficiency compared to traditional processes requiring six to ten months.
- Virtual Research Assistants for R&D Productivity: AI-driven virtual assistants have been adopted to automate routine research duties, including literature searches and draft preparation. These tools summarize datasets, highlight experimental findings, and support documentation, enabling scientists to focus on higher-value tasks and improving productivity within early-stage research environments.
- Synthetic Data Generation to Overcome Data Scarcity: Generative AI models are being used to produce synthetic data where real datasets are limited or noisy. Early applications across pharmaceutical datasets show improved predictive accuracy for toxicity, solubility, and bioactivity, strengthening model performance and supporting better decision-making in data-constrained research settings.
Use Cases
- Accelerating Novel Molecule Discovery: Generative AI models have enabled prediction of biologically active compounds with targeted profiles. Around 30 percent of generated candidates advanced to laboratory validation by 2025, reducing discovery timelines and enabling progression from target identification to hit-finding in under six months.
- Enhancing Clinical Trial Design: Generative AI has been used to simulate patient cohorts and anticipate outcome distributions, improving protocol design. Pilot studies indicate trial enrollment timelines improved by 20 percent, particularly benefiting rare disease studies where limited patient availability requires more efficient recruitment strategies.
- Overcoming Limited Data in Early Research: Synthetic data generation has been applied to small-scale studies to strengthen predictive modeling when real measurements are limited. Across six pilot datasets, synthetic augmentation improved accuracy for key molecular properties by about 15 percent, supporting earlier prioritization of high-value candidates.
Frequently Asked Questions on Generative AI in Pharmaceutical
- What is generative AI in the pharmaceutical industry?
Generative AI in the pharmaceutical industry refers to advanced algorithms designed to create molecular structures, predict drug responses, and support decision-making. Its use enables faster discovery, reduced development timelines, and improved research accuracy across multiple stages of drug development. - How is generative AI used in drug discovery?
Generative AI is applied to design novel molecules, simulate biological interactions, and identify high-potential candidates. These capabilities allow early-stage research to progress more efficiently by decreasing manual screening efforts and improving the precision of therapeutic target identification. - What benefits does generative AI provide to pharmaceutical companies?
The use of generative AI provides benefits such as accelerated innovation, reduced development costs, and enhanced prediction accuracy. These improvements support stronger R&D productivity, lower project risks, and faster translation of scientific insights into viable therapeutic solutions. - Which technologies dominate the generative AI in pharmaceutical market?
Deep learning models currently dominate the technology landscape due to their strong predictive capabilities and wide applicability. Their use supports complex data interpretation, molecular modeling, and the generation of high-quality outputs that assist in drug discovery and clinical research processes. - Which segment holds the largest share in generative AI methods?
Text generation holds the largest market share because it assists with literature analysis, documentation, and scientific data processing. Its capability to streamline research workflows positions it as a key method used across pharmaceutical applications. - Which application area leads the generative AI pharmaceutical market?
Research and development leads the market due to its significant reliance on computational models for molecule prediction, hypothesis testing, and data interpretation. Generative AI supports faster experimentation cycles and improves the reliability of early scientific assessments. - Why is North America leading the generative AI in pharmaceutical market?
North America leads because it hosts advanced research institutions, major pharmaceutical companies, and strong AI technology providers. Supportive regulatory actions and high investment levels further strengthen the region’s leadership in adopting generative AI solutions.
Conclusion
The integration of generative artificial intelligence is expected to remain a transformative force in the pharmaceutical sector, as its ability to accelerate discovery, enhance prediction accuracy, and improve operational efficiency becomes more widely recognized.
Market expansion is supported by strong technological adoption, rising investment, and evolving regulatory frameworks that encourage responsible implementation. Advancements in molecular design, clinical development, and data augmentation continue to strengthen R&D productivity and reduce development timelines.
With North America leading adoption and global interest increasing, generative AI is projected to play a central role in shaping next-generation pharmaceutical innovation and patient-focused outcomes.


