Table of Contents
Overview
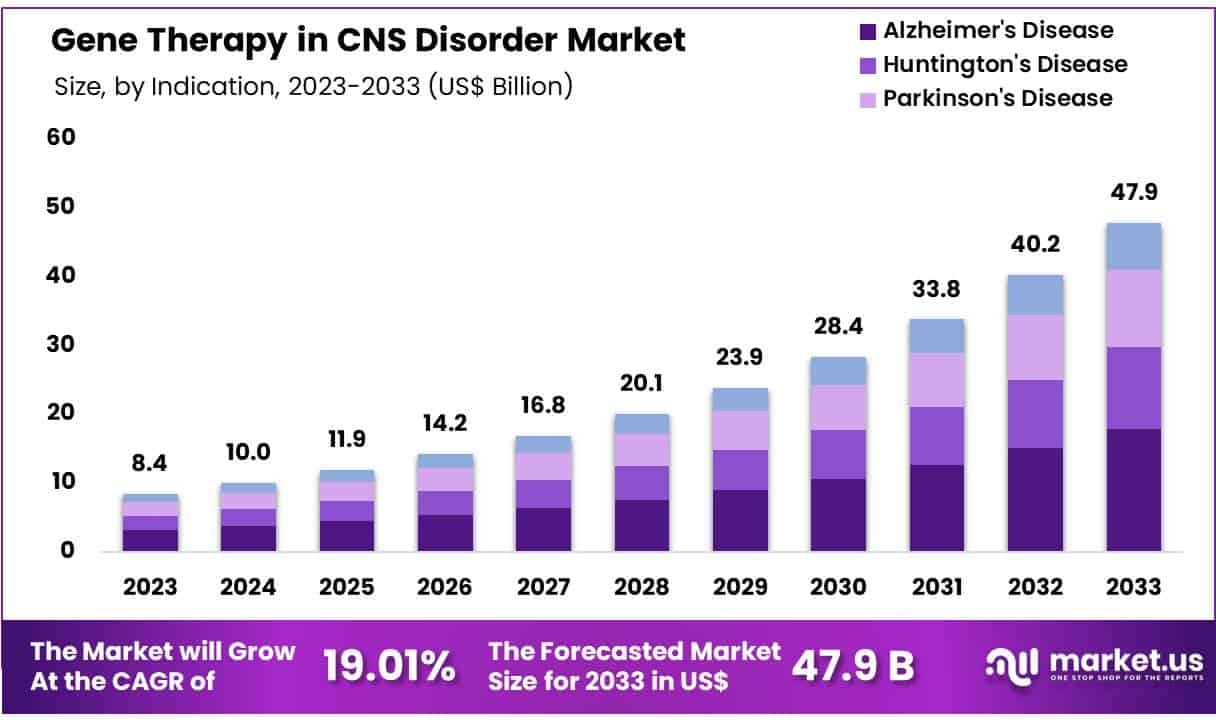
New York, NY – May 19, 2025 – Global Gene Therapy in CNS Disorder Market size is expected to be worth around US$ 47.9 Billion by 2033 from US$ 8.4 Billion in 2023, growing at a CAGR of 19.01% during the forecast period from 2024 to 2033.
The market expansion is primarily driven by the increasing prevalence of neurological disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and rare genetic conditions like Batten disease. Gene therapy offers targeted treatment by modifying or replacing faulty genes, representing a transformative approach in neurological care.
Technological advancements in gene-editing tools like CRISPR, growing investment in biotechnology, and supportive regulatory policies are enhancing research and development in the CNS therapy space. In Vivo gene therapy, delivering genes directly to the brain or spinal cord, currently holds the largest market share due to its efficacy in treating CNS-related conditions.
North America dominates the global market, accounting for the largest share in 2023, owing to high disease prevalence, advanced healthcare infrastructure, and increased funding for neurological research. As clinical trials advance and more gene therapies receive regulatory approvals, the CNS disorder gene therapy market is expected to witness substantial innovation and global adoption in the coming years.
Key Takeaways
- Market Size: The global Gene Therapy in CNS Disorder Market was valued at USD 8.4 billion in 2023 and is projected to reach approximately USD 47.9 billion by 2033.
- Market Growth: The market is expected to grow at a compound annual growth rate (CAGR) of 19.01% from 2024 to 2033.
- Indication Analysis: Among various indications, Alzheimer’s disease led the market in 2023, accounting for a 37.4% share of the total revenue.
- Type Analysis: By therapy type, In Vivo gene therapy was the dominant approach in 2023, holding a 53.2% market share.
- End-Use Analysis: Hospitals emerged as the primary end-user segment, representing 64.9% of the total market share in 2023.
- Regional Analysis: North America is expected to remain the leading region, projected to hold approximately 38.3% of the market share during the forecast period.
Segmentation Analysis
- Indication Analysis: Alzheimer’s disease held a dominant 37.4% market share in 2023 within the CNS gene therapy space. This is attributed to its high global prevalence, rising elderly population, and lack of curative treatments. Significant investment and advancements in targeted gene delivery further support this leadership. Huntington’s and Parkinson’s diseases are also growing, driven by gene-editing innovations and dopamine-targeted therapies. Batten disease, though rare, is gaining momentum through clinical trials and orphan drug support initiatives.
- Type Analysis: In Vivo gene therapy led with a 53.2% share in 2023 due to its direct delivery of genetic material into the brain or spinal cord using viral vectors. This approach offers efficient, targeted treatment essential for CNS disorders. Ex Vivo therapy, involving the external modification and reinfusion of patient cells, is expanding, especially in Huntington’s and Batten disease research. Though smaller, its growth is supported by its customization potential and promising outcomes in ongoing trials.
- End User Analysis: Hospitals dominated the end-user segment in 2023 with a 64.9% share, serving as primary hubs for gene therapy due to their infrastructure, equipment, and specialist teams. These facilities handle complex treatments for major CNS disorders such as Alzheimer’s, Parkinson’s, and ALS. Specialty clinics, though smaller, are gaining traction by offering targeted, condition-specific care. Their collaborations with research entities are expected to enhance gene therapy accessibility and drive future growth in rare CNS disorder treatments.
Market Segments
Indication
- Alzheimer’s Disease
- Huntington’s Disease
- Parkinson’s Disease
- Batten Disease
Type
- Ex-Vivo
- In Vivo
End User
- Hospitals
- Speciality Clinics
Regional Analysis
North America is projected to capture approximately 38.30% of the gene therapy in CNS disorder market during the forecast period. This dominance is supported by a high prevalence of neurological conditions such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. The region benefits from an advanced healthcare infrastructure, robust R&D activities, substantial investment, and a favorable regulatory framework.
The United States leads the regional market, driven by rising mortality from CNS disorders and a growing demand for innovative therapies. The presence of leading pharmaceutical companies, high healthcare spending, and a large patient base further contribute to market expansion. Canada follows as a key contributor, supported by national research initiatives and access to advanced therapeutic options.
Emerging Trends
- Refined Viral Vectors for Blood–Brain Barrier Penetration: The design of adeno-associated virus (AAV) vectors has been optimized to cross the blood–brain barrier more efficiently. Serotypes such as AAV9 and engineered variants (e.g., PHP.B) are now routinely used in preclinical and early-phase clinical studies, enhancing gene delivery to neurons and glial cells.
- Rise of Precision Base and Prime Editing: CRISPR-based base editors and prime editors are being deployed to correct point mutations in single treatments. In one high-profile case, a personalized CRISPR base-editing therapy was designed, manufactured, and administered within six months for a baby with CPS1 deficiency, demonstrating the feasibility of ultra-rapid, patient-specific treatments.
- Surge in Early-Phase Clinical Trials: The number of registered clinical trials for CNS gene therapies has increased sharply. At least 30 active U.S. studies are now evaluating brain-delivered gene therapies for disorders ranging from Parkinson’s disease to Huntington’s disease and rare metabolic conditions.
- Non-Viral Delivery Strategies: Lipid nanoparticle (LNP) platforms—already proven in mRNA vaccines—are being adapted to transport CRISPR components and therapeutic RNAs into the CNS. Early animal studies suggest that LNPs can achieve widespread gene editing in the brain without surgical administration.
- Expanded Public Funding and Collaborative Networks: In May 2025, the NIH launched the Ultra-Rare Gene-based Therapy (URGenT) Network to support development of gene therapies for ultra-rare neurological diseases. This program will fund multi-institutional consortia, facilitating shared resources and data for diseases affecting very small patient populations.
Use Cases
- CRISPR Base Editing for CPS1 Deficiency: A single infant with carbamoyl-phosphate synthetase 1 (CPS1) deficiency received a bespoke CRISPR base-editing treatment. Within ten months post-therapy, the child met key developmental milestones and tolerated a more normal protein-rich diet, illustrating how rapid, personalized gene editing can address life-threatening CNS metabolic disorders.
- Aromatic L-Amino Acid Decarboxylase (AADC) Deficiency: Brain-targeted gene replacement for AADC deficiency—a disorder causing severe movement and autonomic dysfunction—has entered clinical evaluation in over 30 U.S. trials. Early data indicate sustained increases in cerebral AADC enzyme activity and motor improvements in treated patients.
- Onasemnogene Abeparvovec for Spinal Muscular Atrophy (SMA): Although SMA primarily affects motor neurons, it exemplifies CNS-directed gene therapy. Onasemnogene abeparvovec was first approved for infants in 2019, and its Biologics License Application was supplemented on February 5, 2025 to expand age and dosing parameters. This therapy has set a regulatory precedent for viral-vector CNS treatments.
- AAV-Mediated ASPA Gene Delivery in Canavan Disease: AAV-based gene therapy trials for Canavan disease began in 2002. In one human subject followed for five years post-treatment, normalization of key brain metabolites and improved neurological function were observed, demonstrating durability of CNS gene replacement approaches.
Conclusion
The gene therapy market for CNS disorders is experiencing significant expansion, driven by the rising burden of neurological diseases, advances in gene-editing technologies, and increasing clinical trial activity. In Vivo approaches currently dominate, particularly in treating severe conditions such as Alzheimer’s and Parkinson’s.
North America remains the leading region due to its robust healthcare infrastructure and regulatory support. Innovations such as refined viral vectors, precision editing, and non-viral delivery methods are accelerating therapeutic development. With strong public funding and promising clinical outcomes, gene therapy is poised to transform neurological care and offer hope for previously untreatable CNS conditions.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



