Table of Contents
Introduction
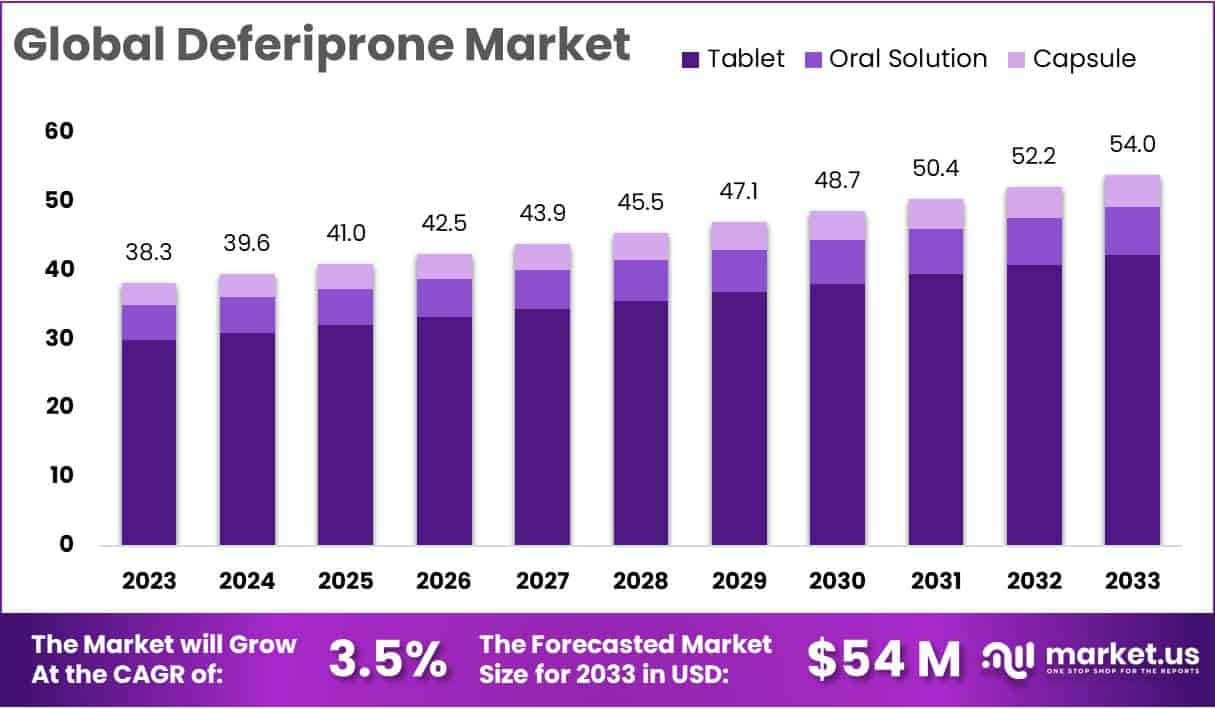
The Global Deferiprone Market was valued at approximately USD 38.3 million in 2023 and is projected to reach around USD 54 million by 2033. This indicates a steady compound annual growth rate (CAGR) of 3.5% over the forecast period. The consistent rise is supported by increasing awareness, better healthcare infrastructure, and expanding access to treatment in both developed and developing nations. Demand for iron chelation therapy continues to grow due to a rising patient population that requires long-term management of iron overload disorders.
The growth of the Deferiprone market can be attributed primarily to the increasing prevalence of thalassemia and other transfusion-dependent blood disorders. According to the World Health Organization (WHO), millions of individuals globally are affected by these conditions, especially in South Asia, the Middle East, and the Mediterranean. Regular blood transfusions in these patients result in excess iron accumulation, which must be managed through chelation therapy. Deferiprone, being an effective oral iron chelator, has become a preferred treatment option, contributing significantly to market demand.
Public health initiatives and newborn screening programs have positively impacted market dynamics. In countries like the U.S., government bodies such as the Centers for Disease Control and Prevention (CDC) support early diagnosis of genetic disorders, increasing the need for lifelong treatments such as Deferiprone. In India, national programs by the Ministry of Health and Family Welfare provide financial assistance and access to iron chelation therapy. These initiatives have expanded access to treatment in middle-income countries, enhancing market penetration and long-term growth potential.
Deferiprone’s convenience as an oral therapy provides a significant advantage over injectable alternatives. It promotes higher patient adherence and quality of life, improving treatment outcomes. Regulatory support has also enhanced the market landscape. Health authorities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved Deferiprone for multiple iron overload indications. These approvals have not only widened its therapeutic use but have also increased physician confidence, strengthening the market position of the drug.
Rising survival rates among patients with transfusion-dependent conditions have led to growing demand for long-term iron chelation therapy. As treatment outcomes improve, patients require continuous care to manage iron levels. This has sustained the usage of Deferiprone over extended periods. Furthermore, ongoing clinical research is exploring new indications for the drug, which is expected to create future growth opportunities. Increased availability, regulatory backing, and better compliance with oral medications are expected to further support the market in the coming years.
Key Takeaways
- An industry analyst highlighted that the Deferiprone market is projected to reach USD 54 million by 2033, growing at 3.5% annually.
- In 2023, tablets accounted for 78.6% of the market, significantly ahead of oral solutions and capsules in global Deferiprone formulations.
- Iron Overload Disorders secured a leading 37.5% share in 2023, primarily influenced by the growing incidence of conditions like hemochromatosis.
- According to recent data, Transfusional Iron Overload represented 61.1% of the indication share in 2023, maintaining a stronghold in treatment focus.
- Market experts attribute growth to increasing cases of iron disorders and sustained research and development efforts in chelation therapy.
- Despite growth, adverse side effects, patient non-compliance, and strict regulatory barriers continue to restrain the market’s full potential.
- Emerging markets present new avenues for growth, with strategic collaborations, pediatric use, and personalized medicine offering untapped potential.
- Current trends include a shift toward oral chelation therapies, enhanced patient engagement, and innovative drug delivery technologies in treatment models.
- In 2023, North America held over 39.1% of the market, driven by rising iron disorder cases and a well-established healthcare framework.
- Experts foresee steady evolution supported by healthcare innovations, regulatory alignment, and increased market activity in developing regions and partnerships.
Segmentation Analysis
Type Analysis
In 2023, tablets held a dominant 78.6% share in the Deferiprone market. This leadership is attributed to their ease of use and portability. The tablet form is widely accepted by consumers due to its convenient administration. It remains the preferred option across various demographics. The oral solution segment followed, offering a user-friendly liquid format. It appealed to patients needing alternative intake methods. Capsules, though smaller in share, showed consistent growth. This reflects a niche demand for encapsulated formulations. These trends highlight diverse consumer preferences influencing market segmentation.
Therapeutic Use Analysis
The Deferiprone market saw Iron Overload Disorders leading in 2023, with a 37.5% market share. This dominance was driven by the growing cases of hemochromatosis and related conditions. The drug’s effectiveness in reducing iron buildup supported this trend. Thalassemia Treatment also held a significant role. Its hereditary nature and regular transfusion needs increased demand for Deferiprone. Additionally, Sickle Cell Disease Treatment contributed notably. It underscored the drug’s efficacy across multiple applications. These therapeutic segments show Deferiprone’s wide utility in managing chronic iron overload disorders in clinical practice.
Indication Analysis
Transfusional Iron Overload led the Deferiprone market in 2023, securing over 61.1% share. This was largely driven by patients undergoing frequent transfusions, especially those with thalassemia. Deferiprone proved effective in managing iron accumulation in such cases. The Non-Transfusion Dependent Thalassemia (NTDT) Caused Overload segment also gained attention. Though smaller in size, it showed growth potential and increasing clinical adoption. These segments together reflect the drug’s adaptability. They also point to a broader recognition of Deferiprone’s role in treating iron overload across both transfusional and non-transfusional patient groups.
Regional Analysis
In 2023, North America held a leading position in the Deferiprone Market, accounting for over 39.1% of the global share. The market reached a valuation of USD 14.9 million. The rising incidence of iron overload disorders such as thalassemia and hemochromatosis has significantly contributed to this growth. These disorders have created a strong demand for Deferiprone as a preferred therapeutic option. The market expansion in the region can also be linked to increased disease awareness and accessibility to treatment options.
The presence of advanced healthcare infrastructure in North America has played a vital role in the widespread adoption of Deferiprone. High diagnostic capabilities and efficient treatment facilities have supported better disease management. Moreover, a strong regulatory framework ensures the safety and efficacy of the drug, which builds trust among healthcare providers. Regulatory approvals and endorsements have encouraged the widespread use of Deferiprone. This favorable environment has strengthened the region’s market position.
North America also benefits from ongoing pharmaceutical R&D and patient education programs. These efforts have led to the development of improved formulations and therapeutic strategies. Public awareness initiatives have helped patients understand iron overload disorders and available treatments. As a result, patient acceptance of Deferiprone has grown steadily. Looking ahead, continued innovation and healthcare improvements are expected to reinforce the region’s leadership. North America is likely to remain a key growth driver in the global Deferiprone Market.
Emerging Trends
- Expanding Therapeutic Applications Beyond Thalassemia: Deferiprone was first approved to manage iron overload in thalassemia major. However, new studies are now exploring its benefits in other conditions involving iron imbalance. Promising research supports its use in rare neurodegenerative disorders like Friedreich’s ataxia. It is also being evaluated for Parkinson’s disease and Neurodegeneration with Brain Iron Accumulation (NBIA). These conditions are characterized by abnormal iron buildup, making Deferiprone a potential treatment. As more clinical trials move forward, its therapeutic scope is expected to widen. The demand for such repurposed drugs is also rising. This trend supports further investment in clinical development beyond traditional uses.
- Growing Preference for Oral Iron Chelators: Oral drugs are preferred over injectables due to ease of use. Deferiprone is taken orally, offering a major advantage over parenteral options like desferrioxamine. This route of administration improves patient comfort and adherence. It is particularly effective in pediatric patients who may resist injections. It is also helpful in low-resource settings where medical infrastructure is limited. With long-term therapy often required, oral chelators are increasingly seen as more practical. This has led to growing clinical and commercial preference. As a result, Deferiprone’s adoption continues to expand across various treatment settings.
- Rising Use in Combination Therapies: Some patients do not respond well to monotherapy with iron chelators. For such cases, combining Deferiprone with other agents has shown better outcomes. Combinations with deferasirox or desferrioxamine are being studied for improved iron removal. This dual approach may enhance efficacy in difficult-to-treat cases. It is particularly relevant for patients with severe or refractory iron overload. Clinical evidence supports its safety and effectiveness in combination regimens. This trend highlights a more personalized approach to treatment. Healthcare providers are now exploring these strategies to optimize results, especially in complex patient profiles.
- Regulatory Approvals and Label Expansion: Deferiprone has secured several important regulatory milestones. It was approved by the U.S. FDA in 2011 for managing thalassemia-related iron overload. Since then, it has also received Orphan Drug Designation for Friedreich’s ataxia. Multiple clinical trials are underway in the U.S. and Europe for new indications. These include phase II and phase III studies targeting rare diseases. As evidence grows, regulatory authorities are expanding its label for broader use. This progress reflects increased confidence in the drug’s safety and efficacy. Such developments are expected to strengthen its market presence globally.
- Increased Focus on Rare Disease Markets: Rare diseases are attracting more research and funding worldwide. Deferiprone is well-positioned in this space as a niche therapy. Its approval for orphan diseases highlights its relevance in targeted markets. Pharmaceutical companies are now investing more in drugs that treat small patient populations. These drugs often receive faster approvals and market exclusivity. Deferiprone’s expanding use in rare conditions supports this trend. It aligns with global strategies to address unmet medical needs. This shift towards orphan drugs is expected to benefit Deferiprone’s growth in the years ahead.
Use Cases
- Iron Overload in Thalassemia: Deferiprone is mainly used to manage iron overload in patients with transfusion-dependent thalassemia. These patients often receive blood transfusions every 2 to 4 weeks, leading to iron buildup in the body. Deferiprone helps remove excess iron by binding to it and allowing its removal through urine. According to the NIH, over 300,000 children are born each year with major hemoglobin disorders, including thalassemia. Clinical trials have shown that Deferiprone can reduce serum ferritin levels by 40–50% over a 12-month period. This reduction helps prevent long-term complications, including liver damage and heart disease, caused by excess iron.
- Iron Overload in Sickle Cell Disease: Sickle cell disease patients frequently need blood transfusions, which increases the risk of iron overload. Deferiprone is used in these patients when other iron chelators are not effective or well-tolerated. It binds to free iron in the bloodstream, helping the body remove it through urine. Deferiprone offers a convenient oral treatment option and is gaining popularity among clinicians for sickle cell patients with high iron levels. Its use is especially beneficial in managing iron-related complications such as liver and heart issues. Regular monitoring is essential to track serum ferritin and maintain safe iron levels during treatment.
- Cardiac Iron Overload: Excessive iron can build up in the heart, leading to cardiac complications. This is a serious risk for patients who rely on regular blood transfusions. Deferiprone has shown superior results in removing iron from the heart compared to other iron chelators. Reducing cardiac iron is essential for preventing heart failure, a leading cause of death in transfusion-dependent patients. Clinical evidence supports Deferiprone’s ability to improve cardiac MRI T2* values, a key marker of heart iron. With long-term use, patients experience better heart function and improved survival rates. Deferiprone is often combined with other therapies for optimal results.
- Neurodegenerative Disorders: Deferiprone can cross the blood-brain barrier, which makes it a candidate for treating brain-related iron accumulation. It is being studied in conditions such as Parkinson’s disease, Alzheimer’s disease, Friedreich’s ataxia, and NBIA (Neurodegeneration with Brain Iron Accumulation). In Friedreich’s ataxia, for example, iron builds up in mitochondria, leading to nerve damage. Early research shows that Deferiprone can reduce this iron and improve neurological scores after six months of use. While results are still under investigation, its ability to reach brain tissue offers hope for future therapies targeting iron-related neurodegeneration.
- Off-Label and Experimental Uses: Beyond approved uses, Deferiprone is also being studied in several experimental areas. These include Hepatitis C-related iron overload, myelodysplastic syndromes, and some types of cancer. In cancers, Deferiprone may work by disrupting iron metabolism in tumor cells. For instance, in preclinical studies on hepatocellular carcinoma, Deferiprone was found to suppress tumor cell growth. Researchers are exploring its potential as a supportive treatment in oncology. Although not yet approved for these uses, early findings suggest Deferiprone could play a broader role in managing diseases linked to abnormal iron metabolism.
Conclusion
In conclusion, the Deferiprone market is showing steady growth due to increasing awareness of iron overload disorders and the drug’s expanding therapeutic use. Its oral formulation makes it easier for patients to take, leading to better treatment outcomes and long-term use. Ongoing research into new applications, such as in neurodegenerative diseases, highlights its future potential. Support from regulatory bodies and public health programs is also helping improve access, especially in developing regions. Despite some challenges like side effects and strict regulations, the overall outlook remains positive. With continued innovation and broader clinical acceptance, Deferiprone is expected to maintain a strong position in the global healthcare landscape.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



