Table of Contents
- Introduction
- Editor’s Choice
- History of Deep Brain Stimulation Statistics
- Global Neurostimulation Devices Market Size
- Deep Brain Stimulation Devices Market Statistics
- Implementation of Deep Brain Stimulation Procedures Statistics
- Discharge Outcomes for Deep Brain Stimulation Patients – By Indication Statistics
- Demographics and Clinical Characteristics of Patients with Parkinson’s Disease Undergoing DBS
- Cost Metrics of Different Devices
- Approval of New Devices
- Key Spending and Investment Statistics
- Innovations and Developments in Deep Brain Simulation Technology Statistics
- Regulations for Deep Brain Simulation Devices Statistics
- Recent Developments
- Conclusion
- FAQs
Introduction
Deep Brain Stimulation Statistics: Deep Brain Stimulation (DBS) is a neurosurgical procedure that involves implanting electrodes in specific brain regions to deliver electrical impulses, helping to modulate abnormal brain activity.
It is primarily used to treat neurological disorders like Parkinson’s disease, essential tremor, dystonia, OCD, epilepsy, and refractory depression.
DBS targets areas such as the subthalamic nucleus or globus pallidus to alleviate motor symptoms, reduce tremors, and improve quality of life.
While it offers significant benefits, including reduced medication dependency, it carries risks such as infection, device malfunction, and potential side effects like mood changes. Research is ongoing to explore its efficacy in additional conditions.

Editor’s Choice
- The modern iteration of DBS began in 1947 with the introduction of a stereotactic apparatus by Spiegel and Wycis, which was initially used to localize areas for brain ablation procedures and to minimize side effects during neurosurgery.
- By 2033, the global DBS devices market revenue is expected to reach USD 3.5 billion.
- The global Deep Brain Stimulation (DBS) devices market is highly competitive, with Abbott leading the sector, holding a 15% market share.
- The regional distribution of the global Deep Brain Stimulation (DBS) devices market in 2021 reveals a significant concentration in North America, which accounted for 53.6% of the market share.
- Among Parkinson’s disease (PD) patients who underwent Deep Brain Stimulation (DBS), the age group distribution reveals that the majority were diagnosed between the ages of 50 and 59, comprising 37.9% of cases.
- In 2018, U.S. neurostimulation generator prices varied by category, with the epilepsy segment seeing the highest increase at 10.3%, driven by rising demand or technological advancements.
- The FDA grants Breakthrough Device Designation to certain DBS systems, like those for treating Alzheimer’s disease, which expedites their development and review processes due to their potential to provide more effective treatment for life-threatening or irreversibly debilitating diseases.
History of Deep Brain Stimulation Statistics
- Deep Brain Stimulation (DBS) has evolved significantly from its early origins in therapeutic brain stimulation.
- The modern iteration of DBS began in 1947 with the introduction of a stereotactic apparatus by Spiegel and Wycis, which was initially used to localize areas for brain ablation procedures and to minimize side effects during neurosurgery.
- DBS technology was further refined through the pioneering work of José Delgado in the 1950s, who experimented with implanted electrodes in humans and animals, leading to the use of DBS for treating neurological and psychiatric conditions with remote-controlled stimulation.
- Over the decades, advancements in neuroimaging, surgical techniques, and a better understanding of brain function have expanded the applications of DBS.
- It has become a crucial treatment for movement disorders such as Parkinson’s disease, as well as offering potential therapies for psychiatric disorders.
- The technology has continually adapted, featuring improvements in electrode design, battery life, and stimulation methods, aiming to enhance patient outcomes and reduce side effects.
(Source: Frontiers)
Global Neurostimulation Devices Market Size
- The global neurostimulation devices market demonstrated significant growth between 2018 and 2019, with market size increasing from USD 6.8 billion in 2018 to USD 7.7 billion in 2019.
- This growth reflects advancements in neurostimulation technologies and increased demand for minimally invasive treatments for neurological disorders.
- Projections indicate that the market will continue its upward trajectory, reaching an estimated USD 12 billion by 2024.
- This expansion is driven by factors such as the rising prevalence of chronic pain and neurological conditions, technological innovations, and growing healthcare expenditure across key regions.
(Source: Statista)

Neurostimulation Market Distribution – By Supplier
- In 2017, the U.S. neurostimulation market was dominated by Medtronic, which held a commanding 67% market share, reflecting its strong portfolio and leadership in the neurostimulation space.
- Abbott and LivaNova each accounted for 10% of the market, leveraging their innovative product offerings to maintain competitive positions.
- Boston Scientific captured 8% of the market share, driven by its advancements in neurostimulation technology.
- The remaining 5% of the market was distributed among other suppliers, highlighting the presence of smaller players contributing to the overall market dynamics.
(Source: Statista)

Deep Brain Stimulation Devices Market Statistics
Global Deep Brain Stimulation Devices Market Size Statistics
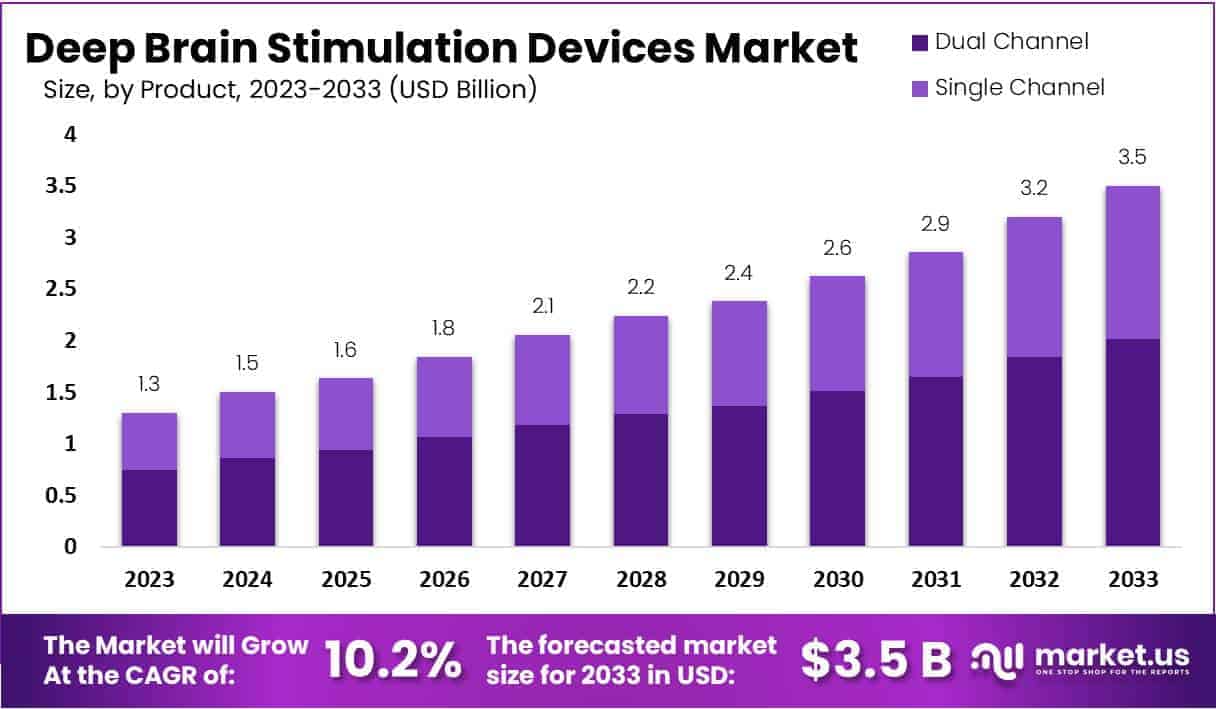
- The global Deep Brain Stimulation (DBS) devices market is projected to experience consistent growth over the forecast period from 2023 to 2033 at a CAGR of 10.2%.
- Starting at a market size of USD 1.3 billion in 2023, the market is expected to reach USD 1.5 billion in 2024, followed by incremental growth to USD 1.6 billion in 2025.
- The upward trend continues, with an estimated market size of USD 1.8 billion in 2026 and USD 2.1 billion in 2027.
- Further growth is anticipated in the latter half of the decade, with the market reaching USD 2.2 billion in 2028 and USD 2.4 billion in 2029.
- By 2030, the market size is projected to rise to USD 2.6 billion, maintaining a positive trajectory with USD 2.9 billion in 2031 and USD 3.2 billion in 2032.
- By 2033, the global DBS devices market is expected to reach USD 3.5 billion, driven by advancements in neurostimulation technologies, increased prevalence of neurological disorders, and a growing acceptance of minimally invasive therapeutic solutions.
(Source: market.us)

Competitive Landscape of Global Deep Brain Stimulation Devices Market Statistics
- The global Deep Brain Stimulation (DBS) devices market is characterized by a competitive landscape dominated by several key players.
- Abbott leads the market with a 15% share, closely followed by Medtronic at 14% and Boston Scientific Corporation at 13%.
- Aleva Neurotherapeutics S.A. holds an 11% market share, while Nexstim and LivaNova PLC each command 9% of the market.
- Neuropace Inc. accounts for 8% of the market, with St. Jude Medical capturing 7%.
- Beijing Pins and SceneRay each hold a modest share of 2%, while other key players collectively represent 10% of the market.
- This competitive distribution reflects a dynamic industry with both established leaders and emerging innovators driving technological advancements and market growth.
(Source: market.us)

Deep Brain Stimulation Devices Market Share – By Region Statistics
- The regional distribution of the global Deep Brain Stimulation (DBS) devices market in 2021 reveals a significant concentration in North America, which accounted for 53.6% of the market share.
- This dominance is attributed to advanced healthcare infrastructure, high adoption rates of neurostimulation technologies, and substantial R&D investments.
- The Asia-Pacific (APAC) region followed with 22.0%, driven by increasing healthcare expenditure and rising awareness of neurological disorders.
- Europe held a 17.0% share, reflecting steady growth due to supportive regulatory frameworks and an aging population.
- South America accounted for 5.0% of the market, while the Middle East and Africa (MEA) region contributed 2.4%, highlighting emerging opportunities for market penetration and growth in these areas.
(Source: market.us)

Implementation of Deep Brain Stimulation Procedures Statistics
- Between 1996 and 2017, a total of 104,356 hospital discharges in the U.S. were associated with the procedure code for neurostimulator lead implantation.
- Of these, 72,427 discharges were for deep brain stimulation (DBS), while 24,865 were for epilepsy and related disorders.
- Additionally, 2,925 discharges were revision procedures, and 4,139 were for unrelated primary diagnoses. It is noteworthy that no DBS-related discharges were recorded from 1993 to 1995.
- Among DBS procedures, the most common reasons were Parkinson’s disease (PD), accounting for 67.2% of cases, essential tremor (ET) at 23.8%, and dystonia at 3.8%.
- In the DBS patient group (excluding epilepsy cases), about 24.7% of admissions (19,673 out of 79,491) included the implantation of an implantable pulse generator (IPG) during the same hospital stay.
- In contrast, only 8.2% of epilepsy-related admissions (1,941 out of 23,633) had concurrent IPG implantation.
- Furthermore, robotic surgery was used in 6.3% (825 out of 12,915) of neurostimulator-related admissions.
- For revision procedures, the majority (86.4% or 2,528 out of 2,925) were due to mechanical complications, such as device malfunctions. Infections accounted for 5.3% (156 cases), while 1.2% (35 cases) were due to surgical wound disruptions.
- The remaining 7.1% (206 cases) were attributed to other complications.
- This data highlights the diverse applications of neurostimulation and the types of challenges faced during and after these procedures.
(Source: Lancet)
Discharge Outcomes for Deep Brain Stimulation Patients – By Indication Statistics
- A comparison of discharge outcomes for Deep Brain Stimulation (DBS) patients with different medical conditions reveals notable trends.
- Among all DBS patients, nearly half (46.9%) were discharged to a nursing facility, intermediate care facility, or another type of care facility.
- This percentage was slightly lower for Parkinson’s disease (PD) patients at 29.9% and for essential tremor (ET) patients at 28.5%.
- For patients with dystonia, epilepsy, and other conditions, the figures were significantly lower, ranging between 4.7% and 5.6%.
- Home health care (HHC) was the second most common discharge destination. About 20.1% of all DBS patients received HHC, with PD and ET patients at 20.3% and 14.4%, respectively.
- Notably, dystonia patients were more likely to receive HHC (9.7%) compared to those with epilepsy (5.7%) and other conditions (6.3%).
- A small fraction of patients left the hospital against medical advice (0.5%), with similar rates observed across PD and ET patients. At the same time, no cases were recorded for dystonia, epilepsy, or other conditions.
- Mortality rates also varied; 3.5% of all DBS patients passed away during their hospital stay, compared to 1.7% of PD patients and 1.3% of ET patients.
- Only 0.1% of epilepsy patients died, while no deaths were reported for dystonia or other conditions.
- Lastly, a negligible number of patients (0%) were discharged alive, but their final destination remained unknown across all patient categories.
- These findings highlight the differences in post-surgical care and outcomes depending on the underlying medical condition, indicating a greater likelihood of facility-based care for certain patient groups and a need for tailored discharge planning.
(Source: Lancet)

Demographics and Clinical Characteristics of Patients with Parkinson’s Disease Undergoing DBS
By Gender
- Among patients with Parkinson’s disease (PD) undergoing Deep Brain Stimulation (DBS), there is a slight predominance of women.
- Women account for 53.9% of the patient population, while men represent 46.1%.
- This gender distribution suggests that more women with PD opt for or are recommended DBS treatment compared to men.
- Understanding the demographic breakdown is essential for tailoring patient care and ensuring equitable access to advanced treatments like DBS.
(Source: JKMS)

By Age group – At Diagnosis
- Among Parkinson’s disease (PD) patients who underwent Deep Brain Stimulation (DBS), the age group distribution reveals that the majority were diagnosed between the ages of 50 and 59, comprising 37.9% of cases.
- This is followed by patients diagnosed under the age of 50, accounting for 31%.
- Those aged 60 to 69 make up 26% of the patient population, while only 5.1% of patients were diagnosed between the ages of 70 and 79.
- This data indicates that DBS is more commonly performed in younger to middle-aged PD patients, likely reflecting the age-related onset and progression patterns of the disease, as well as the suitability of younger patients for surgical intervention.
(Source: JKMS)

Age Group at DBS
- Among Parkinson’s disease (PD) patients undergoing Deep Brain Stimulation (DBS), the age at the time of the procedure shows a significant concentration in the 60-69 age group, which constitutes 39% of cases.
- This is followed by patients aged 50-59, representing 31.1% of the total.
- Those over the age of 70 account for 17%, while only 12.9% of DBS procedures were performed on patients younger than 50.
- These figures suggest that the procedure is most commonly performed on individuals in their sixties, likely reflecting both the progression of PD and the clinical criteria for DBS candidacy.
(Source: JKMS)

By Income Level
- Among Parkinson’s disease (PD) patients undergoing Deep Brain Stimulation (DBS), the distribution by income level reveals that the highest percentage of patients (34.8%) come from high-income groups.
- This is followed by 27.9% from low-income groups, indicating that DBS is utilized across diverse economic backgrounds.
- Patients in the upper middle-income category represent 23.6%, while those in the lower middle-income bracket account for 13.7%.
- These figures suggest that while DBS is more accessible to higher-income individuals, significant portions of lower-income patients also undergo the procedure, potentially reflecting insurance coverage or healthcare support systems.
(Source: JKMS)

By Comorbidity
- Among Parkinson’s disease (PD) patients undergoing Deep Brain Stimulation (DBS), various comorbidities are prevalent.
- Hypertension is the most common, affecting 53% of patients, followed closely by dyslipidemia at 52.1%.
- Depression is also highly prevalent, with 51.8% of patients experiencing this condition.
- Diabetes mellitus affects 41.7% of the DBS patient population.
- Osteoporosis is present in 27.7% of cases, while fractures have been reported in 16.2% of patients.
- PD dementia affects 12.4% of the cohort, and pneumonia, a less frequent but significant comorbidity, is seen in 9.8% of cases.
- These findings highlight the complexity of managing PD patients undergoing DBS, as many also deal with multiple chronic conditions.
(Source: JKMS)

Cost Metrics of Different Devices
- As of 2018, the price changes for neurostimulation generators in the U.S. varied across different medical categories.
- The largest increase was observed in the epilepsy category, where prices rose by 10.3%, reflecting heightened demand or advancements in technology.
- Parkinson’s disease saw a moderate price increase of 3.3%, while the urologic category experienced a 2% rise.
- In contrast, the chronic pain management category recorded a slight price decline of 0.5%, potentially due to market competition or cost-reduction initiatives.
- These variations highlight differing market dynamics and technological developments across neurostimulation applications.
(Source: Statista)

Approval of New Devices
United States
- Between 2015 and 2023, the annual number of novel medtech innovations approved by the FDA fluctuated significantly.
- In 2015, there were 82 approvals, which increased steadily over the next few years, reaching 102 approvals in 2018.
- However, a sharp decline followed, with only 66 approvals in 2019.
- The trend recovered in 2020 with 93 approvals but subsequently dropped again to 82 in 2021 and 62 in 2022.
- The most notable spike occurred in 2023, with FDA approvals reaching 113, marking the highest number in this period.
- These fluctuations reflect evolving regulatory dynamics, innovation cycles, and external factors such as the COVID-19 pandemic impacting the medtech industry.
(Source: Statista)

Japan
- Between fiscal years 2013 and 2022, the number of new medical devices approved by Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) varied considerably based on the year of submission.
- The highest number of approvals occurred in 2014, with 95 products approved, followed by 72 in 2013.
- From 2015 onward, the numbers dropped significantly, stabilizing around 29 to 36 approvals annually until 2020.
- The downward trend continued in 2021 with 26 approvals, culminating in just eight approvals in 2022.
- This decline may reflect changes in regulatory processes, submission rates, or shifts in the medical device industry.
(Source: Statista)

Key Spending and Investment Statistics
Global
- Global research and development (R&D) spending in the medical technology sector has consistently increased from 2011 to 2024.
- In 2011, R&D expenditure was $23.4 billion, rising steadily to $24.1 billion in 2012 and $25 billion in 2013.
- This upward trend continued through the decade, reaching $30 billion in 2018 and $31.3 billion in 2019.
- Despite economic uncertainties, R&D investments grew further during the COVID-19 pandemic, hitting $32.8 billion in 2020 and $34.3 billion in 2021.
- By 2022, spending rose to $35.8 billion, with projections for 2023 and 2024 showing continued growth to $37.3 billion and $38.9 billion, respectively.
- This sustained increase underscores the industry’s commitment to innovation and the development of advanced medical technologies.
(Source: Statista)

India
- The proposed investment value in India’s medical and surgical equipment sector has shown significant fluctuations between the financial years 2011 and 2023.
- In FY 2011, the investment value was ₹1.95 billion, which dropped to ₹0.74 billion in FY 2012.
- A remarkable spike was observed in FY 2013, reaching ₹7.1 billion, followed by a sharp decline to ₹1.31 billion in FY 2014.
- Investments remained relatively modest between FY 2015 and FY 2016, at ₹2 billion and ₹2.24 billion, respectively.
- FY 2017 saw a substantial rise to ₹4.71 billion, but this was followed by a sharp decline to ₹0.77 billion in FY 2018 and an all-time low of ₹0.09 billion in FY 2019.
- Investment values rebounded significantly from FY 2020 onwards, peaking at ₹5.56 billion in FY 2021 and slightly tapering off to ₹4.61 billion in FY 2022 and ₹4.54 billion in FY 2023.
- This data reflects evolving trends and fluctuating investor confidence in the sector.
(Source: Statista)

Japan
- Between fiscal years 2014 and 2023, the average research and development (R&D) expenses and capital investments of medical device manufacturers in Japan displayed notable fluctuations.
- In 2014, R&D expenses peaked at ¥778 million, while capital investment was ¥685 million.
- Both figures declined significantly in the following years, reaching ¥510 million for R&D and ¥390 million for capital investment in 2015.
- The downward trend continued until 2016, followed by a slight recovery in 2017 when R&D expenses rose to ¥595 million and capital investment to ¥403 million.
- In 2018, the expenses remained relatively stable, with R&D at ¥459 million and capital investment at ¥404 million.
- A notable rise occurred in 2019, with R&D expenses at ¥587 million and capital investment at ¥553 million.
- However, 2020 saw an extraordinary spike in R&D expenses, surging to ¥2,039 million, while capital investment fell to ¥330 million.
- From 2021 onwards, both R&D and capital investment stabilized, with moderate levels recorded—¥468 million and ¥429 million, respectively, in 2021.
- By 2023, both metrics had decreased, with R&D expenses at ¥339 million and capital investment at ¥335 million.
- These variations reflect changing priorities and economic factors affecting Japan’s medical device industry over the past decade.
(Source: Statista)

China
- Between 2016 and 2019, the medical device sector in China witnessed fluctuations in the total number of investment deals.
- In 2016, there were 169 deals, which increased to 185 in 2017.
- This upward trend continued into 2018, peaking at 205 investment deals, indicating strong investor interest in the sector.
- However, the number of deals declined significantly in 2019, falling to 151.
- This decline may reflect market saturation, changing regulatory landscapes, or shifts in investor focus.
- Overall, the data highlights dynamic investment activity within China’s medical device industry during this period.
(Source: Statista)

United States
- Between 2019 and 2024, the number of venture investments in the U.S. medical technology industry exhibited significant variability across different funding round sizes.
- In 2019, the majority of investments were under $50M, totaling 449 deals, with 48 deals in the $50–99M range and 18 deals exceeding $100M.
- In 2020, venture activity increased across all categories, with 581 deals under $50M, 71 deals between $50-99M, and a sharp rise to 53 deals exceeding $100M.
- This growth continued in 2021, reaching a peak of 703 deals under $50M and 131 deals in the $50–99M range, though deals over $100M fell to 30.
- In 2022, the number of smaller deals under $50M slightly declined to 613, while deals over $100M reached a high of 57.
- By 2023, there was a further decrease across all categories, with 504 deals under $50M, 55 in the $50–99M range, and 37 deals above $100M.
- The trend continued downward in 2024, with only 289 deals under $50M, 42 in the $50-99M range, and 22 exceeding $100M.
- This data indicates fluctuating investment patterns, potentially influenced by market conditions and evolving industry priorities.
(Source: Statista)

Innovations and Developments in Deep Brain Simulation Technology Statistics
- Recent innovations and developments in Deep Brain Stimulation (DBS) technology have significantly advanced the treatment of neurological disorders.
- In 2023, the field witnessed substantial progress, particularly with the introduction of advanced DBS devices and software, which enhance the precision of stimulation and patient outcomes.
- For instance, Boston Scientific received FDA approval for its new image-guided programming software for DBS, enhancing the accuracy and effectiveness of treatments.
- Concurrently, adaptive DBS systems, which adjust stimulation based on real-time neuronal feedback, reported improved patient experiences and are on the brink of receiving CE marks in the EU.
- Furthermore, novel applications like Stanford Medicine’s use of DBS in treating cognitive impairments from traumatic brain injuries show promising results, offering restored cognitive functions to participants years after their initial injuries.
- Additionally, MIT has introduced a new ultrasound-based approach to brain stimulation, which aims to overcome the limitations of traditional electrical DBS by using sound waves to activate neurons with high precision.
- This ultrasound technique represents a significant shift towards less invasive and more targeted DBS therapies, potentially broadening the scope of treatable conditions and reducing side effects.
- These technological advancements underscore a broader trend in the healthcare sector towards more personalized and efficient neurostimulation therapies, reflecting a shift in how medical professionals approach the treatment of complex neurological disorders.
- Each of these developments not only improves patient care but also expands the potential applications of DBS technology in clinical settings.
(Source: Boston Scientific, Ieee Embs, Mit News, Stanford Medicine)
Regulations for Deep Brain Simulation Devices Statistics
- Deep Brain Stimulation (DBS) devices, regulated as Class III (high-risk) medical devices by the U.S. FDA, must meet stringent premarket approval (PMA) requirements.
- The process for bringing a DBS device to market involves several regulatory steps, starting with the classification of the device based on its risk level, followed by the selection of the appropriate premarket submission—usually a PMA due to the significant risk associated with neurological devices.
- Companies may also engage in the FDA’s pre-submission program to clarify the necessary steps and data required for approval, which is especially pertinent for devices like DBS systems where patient safety and device efficacy are critical.
- Additionally, the FDA grants Breakthrough Device Designation to certain DBS systems, like those for treating Alzheimer’s disease, which expedites their development and review processes due to their potential to provide more effective treatment for life-threatening or irreversibly debilitating diseases.
(Sources: FDA, Practical Neurology, Medtech Intelligence)
Recent Developments
Acquisitions and Mergers:
- Medtronic’s Acquisition of Stimgenics: In January 2020, Medtronic acquired Stimgenics, a company specializing in novel spinal cord stimulation waveforms, to enhance its neuromodulation portfolio. This strategic move aimed to integrate advanced stimulation patterns into Medtronic’s existing DBS systems, potentially improving patient outcomes.
Product Launches:
- Boston Scientific’s Vercise Genus DBS System: In January 2021, Boston Scientific launched the Vercise Genus DBS System, designed to treat symptoms of Parkinson’s disease. This system offers enhanced battery longevity and features Bluetooth-enabled programming, providing clinicians with more precise control over therapy.
- Medtronic’s Percept PC Neurostimulator: In June 2020, Medtronic introduced the Percept PC Neurostimulator, the first DBS system with BrainSense technology capable of sensing and recording brain signals while delivering therapy. This innovation allows for more personalized treatment for patients with neurological disorders.
Funding:
- Enspire DBS Therapy’s Series B Financing: In August 2023, Enspire DBS Therapy announced the completion of a $17.6 million Series B financing round. The funds are intended to advance the development of DBS combined with rehabilitation for stroke patients, aiming to improve motor recovery outcomes.
Conclusion
Deep Brain Stimulation Statistics – Deep Brain Stimulation (DBS) has become a transformative treatment for neurological disorders like Parkinson’s disease (PD), essential tremor, dystonia, and epilepsy.
It offers significant symptom relief where medications fall short, improving patients’ quality of life. Advances in technology, such as adaptive stimulation, have enhanced its effectiveness.
Despite risks like surgical complications and hardware issues, DBS adoption is growing due to the increasing prevalence of neurological conditions and technological innovation.
However, challenges such as high costs and limited accessibility persist. Ongoing research aims to expand its applications and improve outcomes, cementing DBS as a critical tool in neurological care.
FAQs
DBS is a surgical procedure used to treat certain neurological disorders. It involves implanting a device that delivers electrical impulses to specific areas of the brain to help manage symptoms.
DBS is commonly used for Parkinson’s disease, essential tremor, dystonia, epilepsy, and obsessive-compulsive disorder (OCD). Research is ongoing for its use in other conditions, such as depression and chronic pain.
DBS works by sending electrical impulses to targeted areas of the brain that are involved in motor control or other functions. These impulses help regulate abnormal brain activity, improving symptoms such as tremors, stiffness, and motor dysfunction.
Candidates for DBS are typically patients with severe symptoms that are not adequately controlled by medications or other therapies. Eligibility is determined by a comprehensive evaluation by a medical team, including neurologists and neurosurgeons.
Like any surgical procedure, DBS carries risks, including infection, bleeding, or hardware-related complications. Some patients may also experience side effects such as mood changes, speech difficulties, or balance issues.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



