Table of Contents
Overview
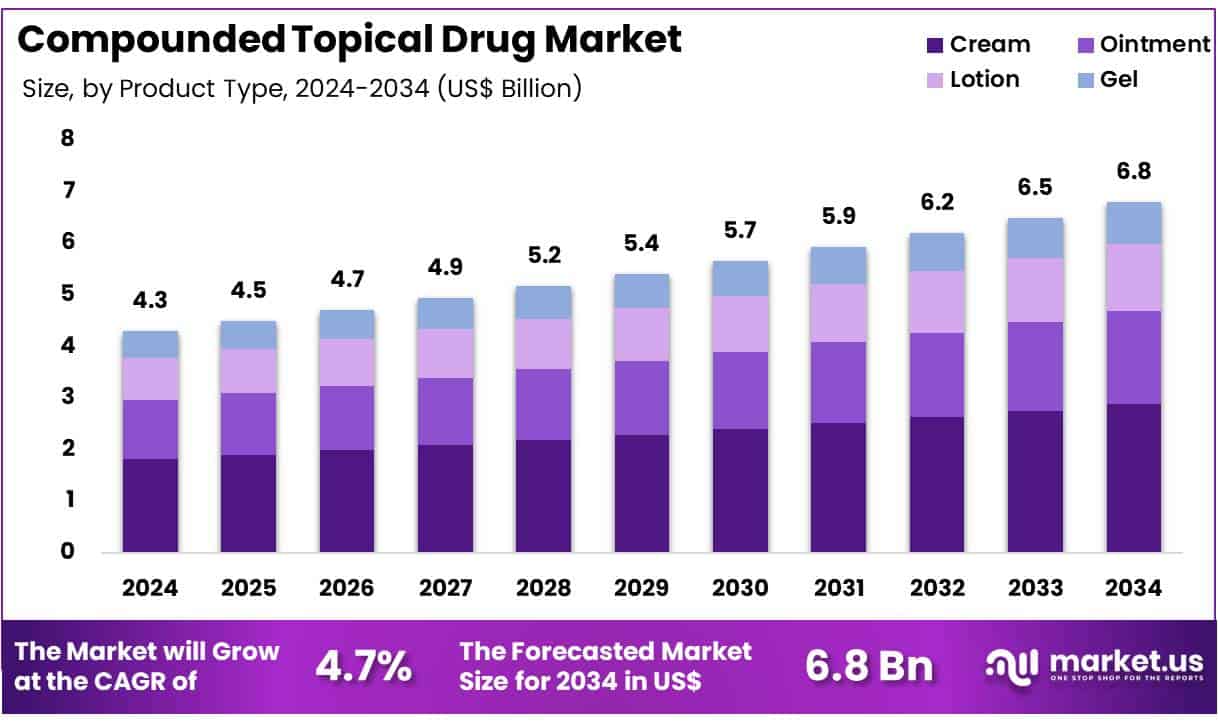
New York, NY – July 03, 2025 – Global Compounded Topical Drug Market size is expected to be worth around US$ 6.8 Billion by 2034 from US$ 4.3 Billion in 2024, growing at a CAGR of 4.7% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 39.8% share with a revenue of US$ 1.7 Billion.
Compounded topical drugs represent a significant advancement in personalized medicine, offering tailored treatments for dermatological, musculoskeletal, and pain-related conditions. These formulations are prepared by licensed pharmacists based on a physician’s prescription, allowing specific combinations of active pharmaceutical ingredients (APIs) in customized dosages, bases, and delivery systems. Such customization enhances therapeutic efficacy and minimizes systemic side effects.
The core of a compounded topical drug formulation typically includes three key components: the active drug(s), the base (or vehicle), and stabilizing excipients. Commonly compounded agents include nonsteroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, anesthetics, and neuropathic pain medications such as gabapentin and amitriptyline. These are often delivered using transdermal creams, gels, ointments, or lotions designed to ensure optimal skin penetration and sustained release.
Bases such as lipoderm, pluronic lecithin organogel (PLO), and hydrophilic gels are selected for their ability to carry APIs across the skin barrier effectively. Stability, bioavailability, and patient tolerability are key parameters considered during formulation.
Compounded topical drugs have gained traction particularly in pain management, podiatry, and veterinary medicine. However, regulatory oversight by the U.S. FDA remains limited to compliance with compounding standards under Section 503A of the Federal Food, Drug, and Cosmetic Act. The growing demand for individualized therapies continues to position compounded topical medications as an important tool in modern clinical care.
Key Takeaways
- In 2024, the global compounded topical drug market generated a total revenue of approximately USD 4.3 billion. The market is projected to expand at a compound annual growth rate (CAGR) of 4.7%, reaching an estimated value of USD 6.8 billion by 2034.
- By product type, the market is segmented into creams, ointments, lotions, and gels. Among these, creams emerged as the dominant category, accounting for a leading market share of 42.3% in 2023. Their widespread preference is attributed to ease of application, high patient compliance, and efficient drug absorption.
- In terms of distribution channels, the market is classified into retail pharmacies, hospital pharmacies, online pharmacies, and others. Retail pharmacies held the largest share, capturing 38.7% of the market, supported by easy accessibility and direct physician-pharmacist collaboration in personalized compounding services.
- Regionally, North America dominated the global market, securing 39.8% of the overall revenue in 2023. This leadership is driven by strong regulatory frameworks, advanced healthcare infrastructure, and increasing demand for customized dermatological and pain management solutions.
Segmentation Analysis
- Product Type Analysis: The cream segment accounted for 42.3% of the compounded topical drug market, driven by its ease of application and patient-friendly texture. Creams offer a non-greasy, fast-absorbing consistency suitable for various skin types, making them ideal for treating conditions like eczema and psoriasis. Their higher bioavailability enhances therapeutic outcomes. With rising demand for personalized dermatological care and dual-purpose formulations offering both therapeutic and cosmetic benefits, compounded creams are expected to witness steady growth throughout the forecast period.
- Application Analysis: Retail pharmacies captured 38.7% of the market share, largely due to increasing patient preference for customized medications. These pharmacies offer convenient access to compounded topical drugs, especially in areas lacking hospital-based compounding services. The adoption of e-prescriptions and patient-driven treatment plans further supports this trend. As more consumers shift away from standard over-the-counter options toward tailored therapies, retail pharmacies are positioned to remain a vital channel in the compounded medication supply chain, supporting ongoing segment growth.
Market Segments
By Product Type
- Cream
- Ointment
- Lotion
- Gel
By Application
- Retail Pharmacy
- Hospital Pharmacy
- Online Pharmacy
- Others
Regional Analysis
North America held the largest share of the compounded topical drug market in 2023, accounting for 39.8% of the total revenue. This dominance is primarily driven by the growing demand for personalized medicine. Physicians in the region are increasingly prescribing customized formulations to meet individual patient needs, including adjusted dosages for pediatric or geriatric populations, allergen-free compounds, and multi-ingredient preparations for improved therapeutic outcomes.
The availability of advanced compounding infrastructure and regulatory support further strengthens the market across the United States and Canada. The rising prevalence of dermatological conditions, chronic pain disorders, and hormone imbalances has enhanced the clinical relevance of tailored topical treatments.
In contrast, the Asia Pacific region is expected to register the fastest compound annual growth rate (CAGR) during the forecast period. This growth is attributed to improvements in healthcare infrastructure, increasing awareness of customized treatments, and the rising incidence of skin-related disorders.
Countries such as China, India, and South Korea are witnessing rapid adoption of compounding practices, supported by a growing base of trained pharmacists. The demand for patient-centric care, coupled with expanding pharmaceutical capabilities, is anticipated to drive strong market development for compounded topical drugs throughout the region.
Emerging Trends
- Growth of Outsourcing Facilities and Regulatory Oversight: The role of large-scale outsourcing facilities has expanded significantly in recent years. Congress created a distinct “outsourcing facility” category under section 503B of the FD&C Act to oversee pharmacies that compound high volumes of medications shipped across state lines. Over the past decade, the FDA has issued more than 60 policy documents (including 27 final guidance documents and 3 final rules) to strengthen compounding quality standards and protect patient safety.
- Advances in Bioequivalence Research: Investment in scientific methods for topical products has increased. The FDA’s generic drug research program now explores novel in vitro (laboratory), in silico (computer modeling), and in vivo (human subject) approaches to establish bioequivalence for compounded dermatological drugs. These efforts aim to make testing more efficient and reliable, reducing reliance on costly clinical studies while ensuring consistent drug delivery through the skin.
- Personalized Medicine and API Diversity: Personalized therapies are driving demand for customized topical formulations. Compounded pain creams commonly contain four to six active pharmaceutical ingredients (APIs) tailored to individual patient needs. In a review of 20 ingredients, 3 APIs (lidocaine, doxepin, naproxen) and one two-drug combination (pentoxifylline + clonidine) showed potential clinical benefit, highlighting a shift toward multi-agent formulations for localized therapy.
- Heightened Safety Surveillance: Adverse event reporting for compounded topicals has increased regulatory scrutiny. For example, 32 cases of adverse events associated with compounded topical finasteride (used off-label for hair loss) were reported to the FDA between 2019 and 2024, prompting safety alerts and provider advisories. This trend underscores growing attention to post-market monitoring of compounded products.
Use Cases
- Pain Management: Compounded topical pain creams are frequently used as adjunct therapy for localized pain. Such creams may include anesthetics (e.g., lidocaine), NSAIDs (e.g., naproxen), and adjuvant agents (e.g., ketamine). In a 2020 National Academies review, it was noted that topical preparations can provide local, regional, or limited systemic analgesia, potentially reducing the need for oral opioids and lowering side-effect risks.
- Dermatological Conditions: Dermatologists have long used compounded formulations to treat conditions not fully addressed by approved products. For example, compounded glycolic acid preparations have been used for epidermal melasma with no major safety concerns in short-term use, and custom tretinoin combinations have been developed for photodamaged skin management over several decades.
- Pediatric and Geriatric Dosage Forms: Compounding enables alternate dosage forms for patients who cannot swallow pills. Liquid topical suspensions or gels can be formulated for pediatric use or for geriatric patients with swallowing difficulties. These adjustments ensure accurate dosing and improve adherence, although systematic data on prescription volume remain limited.
- Hormone Replacement and Dermatologic Aesthetics: Tailored topical hormone gels (e.g., testosterone or estrogen preparations) are prescribed when commercial products are unsuitable or unavailable. Compounded formulations allow for precise dose titration. Additionally, custom blends of active ingredients (e.g., hydroquinone, retinoids) are used in cosmetic dermatology to address pigmentary disorders and fine lines.
- Adverse Event Monitoring: Safety surveillance has identified systemic absorption in topical preparations. Among 24 reported cases involving compounded topical pain creams, 16 were reported by health professionals, and systemic absorption was confirmed in 10 cases via serum or urine testing. This highlights the importance of monitoring compound-specific pharmacokinetics and patient education on proper use.
Conclusion
The compounded topical drug market is gaining prominence as personalized medicine becomes increasingly central to modern healthcare. With growing demand for tailored treatments in dermatology, pain management, and hormone therapy, compounded formulations offer flexibility, improved bioavailability, and patient-specific solutions.
Creams lead in product preference, while retail pharmacies dominate distribution due to accessibility. North America holds the largest market share, while Asia Pacific shows the fastest growth. Advances in regulatory oversight, bioequivalence research, and adverse event monitoring underscore a maturing market. Continued innovation and emphasis on safety will further strengthen the role of compounded topical drugs in targeted therapeutic care.


