Table of Contents
- Introduction
- Editor’s Choice
- Clinical Perinatal Software Market Overview
- Factors Fuelling the Demand for Clinical Perinatal Software
- Causes of Infant Death Detected Through Clinical Perinatal Software
- Key Components in Clinical Perinatal Software
- Key Indicators in Clinical Perinatal Software
- Investments in Clinical Perinatal Software
- Regulations for Clinical Perinatal Software
Introduction
According to Clinical Perinatal Software Statistics, Clinical perinatal software is pivotal in modern obstetric practice, providing healthcare professionals with a comprehensive suite of tools to manage maternal and fetal health data throughout pregnancy, childbirth, and the postpartum period.
This software integrates electronic health record systems tailored to perinatal care, enabling real-time monitoring of vital signs, fetal heart rate, and uterine contractions.
Decision support tools aid in informed clinical decision-making, while customizable workflows streamline documentation processes.
Interoperability ensures seamless information exchange with external systems, and telehealth capabilities facilitate virtual prenatal visits and consultations.
Compliance features uphold regulatory standards, safeguarding patient confidentiality. Altogether, clinical perinatal software enhances efficiency, communication, and patient outcomes in obstetric care settings.
Editor’s Choice
- The global clinical perinatal software market revenue reached USD 287.3 million in 2023.
- In 2033, the market is predicted to achieve USD 808.4 million, with on-premise solutions contributing USD 586.1 million and cloud-based solutions USD 222.3 million.
- The Global Clinical Perinatal Software Market is predominantly driven by integrated solutions, which hold a substantial market share of 84.70%.
- In the Global Clinical Perinatal Software Market, hospitals and clinics dominate the end-use segment, accounting for 63.80% of the market share.
- Significant contributions from several key players characterize the Global Clinical Perinatal Software Market. Leading the market is PeriGen Inc., which holds a 15% share.
- In 2022, the leading causes of infant death in the United States were diverse, with congenital malformations accounting for 19.30% of all infant deaths.
- In the United States, the Centers for Medicare & Medicaid Services (CMS) has implemented electronic clinical quality measures (eCQMs) such as ePC-02 and ePC-07, which focus on cesarean birth rates and severe obstetric complications, respectively.
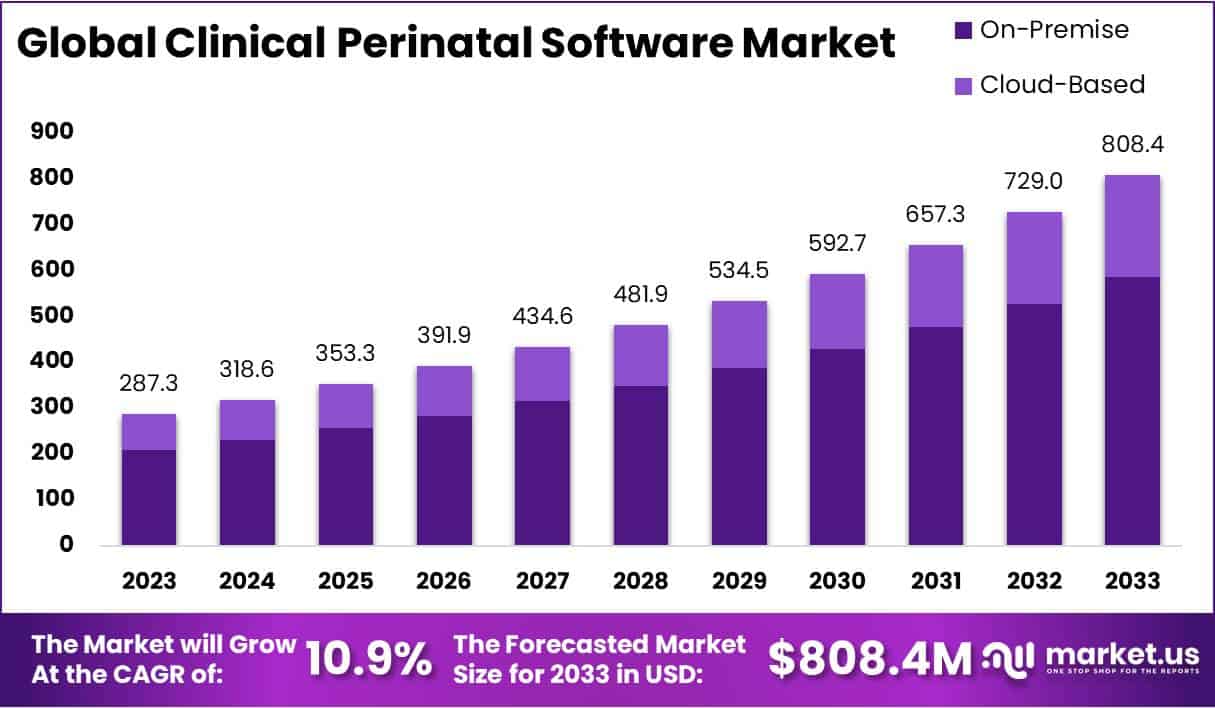
Clinical Perinatal Software Market Overview
Global Clinical Perinatal Software Market Size
- The Global Clinical Perinatal Software Market has exhibited a steady growth trajectory at a CAGR of 10.9%, with revenue increasing from USD 287.3 million in 2023 to a projected USD 808.4 million by 2033.
- The growth momentum is expected to persist, with market revenues reaching USD 592.7 million in 2030, USD 657.3 million in 2031, USD 729.0 million in 2032, and finally culminating at USD 808.4 million in 2033.

Global Clinical Perinatal Software Market Size – By Deployment Mode
- The Global Clinical Perinatal Software Market is projected to grow significantly from 2023 to 2033, segmented by deployment mode into on-premise and cloud-based solutions.
- In 2023, the total market revenue is expected to be USD 287.3 million, with on-premise solutions contributing USD 208.3 million and cloud-based solutions adding USD 79.0 million.
- Finally, in 2033, the market is predicted to achieve USD 808.4 million, with on-premise solutions contributing USD 586.1 million and cloud-based solutions USD 222.3 million.

Clinical Perinatal Software Market Share – By Product Type
- The Global Clinical Perinatal Software Market is predominantly driven by integrated solutions, which hold a substantial market share of 84.70%.
- In comparison, standalone products account for a smaller share of 15.30%.

Global Clinical Perinatal Software Market Share – By End-Use
- In the Global Clinical Perinatal Software Market, hospitals and clinics dominate the end-use segment, accounting for 63.80% of the market share.
- Maternity clinics hold the remaining 36.20% of the market share. These specialized clinics are increasingly adopting perinatal software to enhance maternal and fetal monitoring, streamline administrative tasks, and provide personalized care.

Competitive Landscape of Global Clinical Perinatal Software Market
- Significant contributions from several key players characterize the Global Clinical Perinatal Software Market.
- Leading the market is PeriGen Inc., which holds a 15% share, followed closely by Edan Instruments Inc. with 14% and CooperSurgical Inc. with 13%.
- Koninklijke Philips NV captures an 11% market share, while Cognitive Medical Systems and AS Software Inc. each command a 9% share.
- GE Healthcare holds an 8% share, and Bionet contributes 7% to the market.
- The remaining 14% is occupied by various other key players, collectively making up a substantial portion of the market.

Regional Analysis of the Global Clinical Perinatal Software Market
- The Global Clinical Perinatal Software Market is characterized by a significant regional distribution, with North America leading the market with a substantial 52.0% share.
- Europe follows with a 20.0% share, indicating strong adoption across these regions.
- The Asia-Pacific (APAC) region holds a 15.0% share, reflecting its growing healthcare infrastructure and technological advancements.
- South America accounts for 8.0% of the market, while the Middle East and Africa (MEA) region represents 5.0%.

Factors Fuelling the Demand for Clinical Perinatal Software
Maternal Mortality Statistics
- The countries with the highest maternal mortality rates highlight significant challenges in maternal healthcare.
- South Sudan has the highest rate, with 1,223 maternal deaths per 100,000 live births, followed by Chad at 1,063 and Nigeria at 1,047.
- The Central African Republic also reports a high rate of 835, with Guinea-Bissau at 725 and Liberia at 652.
- Somalia faces a rate of 621, while Afghanistan’s rate is 620.
- Other countries with alarmingly high rates include Lesotho at 566, Guinea at 553, the Democratic Republic of the Congo at 547, Kenya at 530, Benin at 523, and Burundi at 494.

Infant Mortality Statistics
- In 2023, Afghanistan reported the highest infant mortality rate, with 103.06 child deaths per thousand live births.
- Somalia followed with a rate of 85.06, and the Central African Republic recorded 81.74.
- Equatorial Guinea had an infant mortality rate of 77.85, while Sierra Leone reported 72.3.
- Niger and Chad had rates of 65.53 and 63.99, respectively.
- South Sudan recorded 61.63, and Mozambique had a rate of 59.77.
- The Democratic Republic of Congo followed closely with 59.12, and Mali reported 58.99.
- Angola’s infant mortality rate stood at 57.2, with Comoros at 56.01.
- Nigeria and Benin recorded rates of 55.17 and 54.33, respectively, while Côte d’Ivoire had 54.04.
- Pakistan’s rate was 52.73, followed by Mauritania at 49.95.
- Guinea reported an infant mortality rate of 48.32, and Burkina Faso’s rate was 48.17.

Neonatal Mortality Statistics
- In 2022, Sub-Saharan Africa had the highest neonatal mortality rate, with 27 deaths per 1,000 live births.
- Southern Asia followed with a rate of 22, while Northern Africa recorded 15.
- South-Eastern Asia had a neonatal mortality rate of 12, and both Western Asia and Oceania reported rates of 10.
- Central Asia, Latin America, and the Caribbean each had a rate of 9.
- Eastern Asia and Northern America both recorded rates of 3, while Australia and New Zealand, along with Europe, had the lowest rates of 2.

Causes of Infant Death Detected Through Clinical Perinatal Software
- In 2022, the leading causes of infant death in the United States were diverse, with congenital malformations accounting for 19.30% of all infant deaths.
- Low birth weight was the second leading cause, responsible for 14% of infant deaths.
- Sudden infant death syndrome (SIDS) followed, constituting 7.40%, and unintentional injuries accounted for 6.60%.
- Maternal complications contributed to 5.90% of infant deaths, while cord and placental complications were responsible for 3.20%.
- Bacterial sepsis of the newborn caused 3.10% of deaths, and respiratory distress of the newborn was the cause in 2.20% of cases.
- Intrauterine hypoxia and birth asphyxia accounted for 1.80% of infant deaths, and diseases of the circulatory system were responsible for 1.70%.

Key Components in Clinical Perinatal Software
- Key components in clinical perinatal software are designed to enhance maternal and fetal care through comprehensive data integration, real-time monitoring, and advanced analytics.
- These components include maternal-fetal surveillance systems that provide early warning alerts for potential complications, significantly improving patient safety by reducing incidents like uterine tachysystole and unexpected NICU admissions.
- For example, PeriGen’s PeriWatch platform integrates statistical analysis features to offer consistent, real-time data analysis, allowing clinicians to focus more on direct patient care rather than manual data management.
- Additionally, Philips’ IntelliSpace Perinatal system offers a modular design for flexibility and scalability, supporting everything from non-stress tests to full departmental surveillance and charting solutions, ensuring vital signs and alerts are seamlessly integrated and accessible across various platforms.
Key Indicators in Clinical Perinatal Software
- Key indicators in clinical perinatal software are crucial for monitoring and improving maternal and fetal health outcomes.
- The stillbirth rate is calculated by dividing the number of stillbirths by the total number of births (both stillbirths and live births) and is expressed per 1,000 births.
- The percentage of stillbirths that are antepartum is determined by dividing the number of antepartum stillbirths by the total number of stillbirths multiplied by 100.
- The early neonatal mortality rate measures early neonatal deaths (occurring between 1 and 7 days) per 1,000 live births.
- The perinatal mortality rate combines the number of stillbirths and early neonatal deaths divided by the total number of births expressed per 1,000 births.
- Lastly, the neonatal mortality rate accounts for neonatal deaths (occurring between 1 and 28 days) per 1,000 live births.
Investments in Clinical Perinatal Software
- Governments are increasingly funding digital health solutions to enhance maternal and neonatal care, focusing on reducing mortality rates and improving healthcare outcomes.
- For instance, the integration of advanced technologies like artificial intelligence and data analytics into perinatal software is a priority, supported by substantial government grants and initiatives.
- Private sector investments are also robust, with major companies such as Philips Healthcare and PeriGen Inc. leading the charge. These firms are investing heavily in research and development to introduce innovative products and expand their market presence through strategic partnerships and acquisitions.
Regulations for Clinical Perinatal Software
- Regulations for clinical perinatal software vary by country and are designed to ensure the safety, efficacy, and security of these digital health solutions.
- In the United States, the Centers for Medicare & Medicaid Services (CMS) has implemented electronic clinical quality measures (eCQMs) such as ePC-02 and ePC-07, which focus on cesarean birth rates and severe obstetric complications, respectively.
- These measures aim to improve patient outcomes and ensure consistent data collection across healthcare providers. Compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations is also mandatory, ensuring the protection of patient data.
- In Europe, this software must comply with the General Data Protection Regulation (GDPR), which mandates strict data privacy and security measures.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



