Table of Contents
Overview
New York, NY – July 16, 2025: The Global CHAPLE Syndrome Market is projected to reach approximately USD 224.4 million by 2033, up from USD 145.9 million in 2023. This growth represents a steady CAGR of 4.4% during the forecast period from 2024 to 2033. CHAPLE syndrome is a rare immune disorder caused by a deficiency of the CD55 protein. Patients often suffer from protein-losing enteropathy, leading to malnutrition, growth issues, and severe gastrointestinal distress. Early detection, increasing awareness, and advancements in genetic testing are driving new interest in this niche healthcare market.
In August 2023, a major breakthrough occurred with the FDA approval of Veopoz (pozelimab-bbfg), a monoclonal antibody treatment by Regeneron Pharmaceuticals. This was the first targeted therapy for CHAPLE syndrome and has shifted the treatment landscape significantly. Veopoz directly addresses the immune dysfunction associated with CD55 deficiency. The availability of this drug marks a new era for patient outcomes. It also highlights how focused biologics are transforming rare disease care. Approval of such therapies signals growing regulatory support for rare disorder treatments.
Regeneron’s strategic partnership with Orsini Specialty Pharmacy has made Orsini the exclusive distributor of Veopoz. This collaboration supports efficient drug delivery and comprehensive patient management. Orsini’s role includes guiding patients and healthcare providers through the complex treatment process. Specialized pharmacies are playing a critical role in rare disease management. These partnerships ensure continuity of care and tailored support. As rare therapies grow more complex, pharmacy collaboration becomes a core element in market success and patient satisfaction.
Future opportunities in the CHAPLE syndrome market lie in gene therapy and next-generation immune-targeting treatments. Research in these areas is expanding, aiming to offer long-term solutions beyond symptom control. Scientific progress is paving the way for innovative and more precise interventions. Investors and stakeholders are showing increased interest in ultra-rare conditions with high unmet needs. With rising R\&D activity and industry collaboration, the CHAPLE syndrome market is poised for steady growth. The focus remains on improving quality of life through advanced, targeted treatments.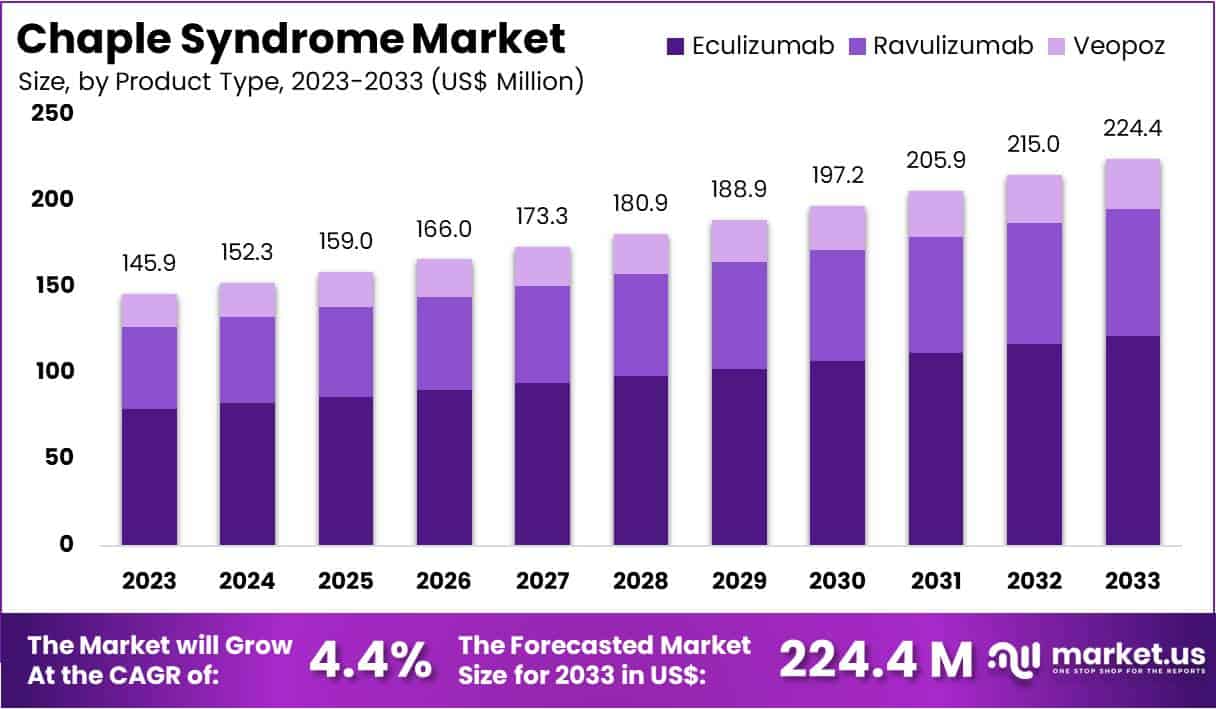
Key Takeaways
- In 2023, the CHAPLE syndrome market generated USD 145.9 million in revenue and is projected to reach USD 224.4 million by 2033.
- The market is growing at a steady CAGR of 4.4% over the forecast period, reflecting increased awareness and new treatment options.
- Among product types, eculizumab dominated in 2023, capturing a significant 54.3% share of the overall market.
- Veopoz and ravulizumab are also emerging, but eculizumab remains the most widely used treatment in current clinical settings.
- By application, hypoalbuminemia was the leading segment, accounting for 38.4% of the market share in 2023.
- Other key applications include gastrointestinal symptoms, malnutrition, edema, and hypogammaglobulinemia, highlighting the syndrome’s diverse clinical impact.
- Hospital pharmacies led the distribution channel category, securing the largest share at 49.2% due to their specialized handling of rare disease medications.
- North America dominated the global market in 2023, with a leading regional share of 39.8%, driven by strong healthcare infrastructure and early drug access.
Regional Analysis
North America currently leads the CHAPLE Syndrome Market, holding the largest revenue share of 39.8% in 2023. This dominance is fueled by advanced healthcare infrastructure, increased funding for rare disease research, and supportive government policies. U.S. government initiatives have strengthened Medicaid and encouraged investment in rare diseases through enhanced funding and legislative support. Additionally, collaborations between pharmaceutical companies and research institutions have advanced CHAPLE-specific therapies, improving patient outcomes. Increased awareness among healthcare professionals has also led to earlier diagnosis and more effective, targeted interventions across the region.
The Asia Pacific region is projected to witness the fastest CAGR during the forecast period, driven by rising awareness of rare diseases and improved access to healthcare services. Governments across countries like China, Japan, and South Korea are actively promoting early diagnosis and treatment for genetic disorders such as CHAPLE syndrome. Investments in healthcare infrastructure and partnerships with international pharmaceutical firms are fostering innovation in localized therapies. Increased healthcare spending and regional efforts to improve patient outcomes are making treatments more accessible, positioning Asia Pacific as a key growth hub for rare disease management.
Segmentation Analysis
In 2023, the eculizumab segment led the CHAPLE syndrome market, capturing a 54.3% share due to its proven efficacy in treating this rare immune disorder. As an FDA-approved complement inhibitor, eculizumab directly addresses the underlying immune dysfunction caused by CD55 deficiency. Its ability to reduce disease symptoms and improve patient outcomes makes it a preferred choice among clinicians. Growing awareness, rising diagnosis rates, and ongoing research to enhance eculizumab’s formulation further support its expanding role as a cornerstone therapy in the CHAPLE treatment landscape.
Among application segments, hypoalbuminemia held the largest share at 38.4%, highlighting its critical role in CHAPLE syndrome progression. This condition, marked by low albumin levels in the blood, is common in affected patients and often leads to severe complications. Improved diagnostic tools are enabling earlier detection and targeted therapeutic interventions. Rising clinical attention, combined with advancements in albumin supplementation and supportive treatments, is expected to sustain this segment’s growth. Continued governmental and institutional support for rare disease care reinforces the need for specialized hypoalbuminemia management strategies.
In terms of distribution, hospital pharmacies dominated with a revenue share of 49.2%, reflecting their central role in rare disease care. These settings are equipped to handle complex drug regimens like eculizumab, requiring precise dosing and close monitoring. Increased hospital admissions related to rare immune disorders and favorable reimbursement policies further boost this channel. Strengthened pharmaceutical supply chains and initiatives to enhance access to high-cost treatments position hospital pharmacies as essential hubs for CHAPLE syndrome therapy distribution and management.
By Product Type
- Eculizumab
- Ravulizumab
- Veopoz
By Applications
- Gastrointestinal Symptoms
- Hypoalbuminemia
- Edema
- Hypogammaglobulinemia
- Malnutrition
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Key Players Analysis
Key players in the CHAPLE syndrome market are focused on developing innovative treatments and strengthening their market position through strategic initiatives. These companies are heavily investing in research and development to create targeted therapies that address the root causes of this ultra-rare genetic disorder. By collaborating with genetic research institutions and healthcare organizations, they are accelerating drug discovery and improving clinical trial outcomes. Expanding into regions with strong rare disease frameworks further enables them to serve affected patient populations more effectively.
In addition to product innovation, major players prioritize raising awareness among healthcare professionals and patients. Educational campaigns and outreach initiatives are helping to improve early diagnosis and promote timely intervention. By enhancing disease understanding across the medical community, these companies aim to improve treatment outcomes and support broader adoption of new therapies. Their multi-pronged approach reflects a long-term commitment to advancing care for individuals living with CHAPLE syndrome.
- Regeneron
- Novartis AG
- Hoffmann-La Roche Ltd
- CinnaGen Co.
- Apellis Pharmaceuticals
- Amgen Inc.
- Akari Therapeutics
Emerging Trends
First Approved Targeted Therapy
In 2023, the approval of Veopoz (pozelimab-bbfg) became a breakthrough for CHAPLE syndrome treatment. It is the first targeted therapy developed specifically for this rare genetic condition. Veopoz helps regulate the immune system by targeting the effects of CD55 deficiency. This approval gives new hope to patients and families affected by CHAPLE. It also encourages pharmaceutical companies to invest in similar rare disease therapies. With this step, the path is open for new innovations, making the treatment journey more effective. The focus has now shifted from managing symptoms to offering more precise medical solutions.
Growing Research in Gene Therapy
CHAPLE syndrome is caused by a mutation in the CD55 gene. Gene therapy is now being studied as a long-term or even permanent treatment option. This approach aims to correct the faulty gene itself, rather than just treating symptoms. Early research shows promise in using advanced gene-editing tools like CRISPR. Scientists believe this method could stop disease progression entirely. Although still in early stages, gene therapy is gaining traction in rare disease treatment. If successful, it may reduce the need for lifelong medications and improve survival and quality of life for CHAPLE patients.
More Clinical Trials for Rare Diseases
There is a growing number of clinical trials focused on ultra-rare diseases like CHAPLE syndrome. These trials aim to test new drugs and understand how the disease behaves in different age groups. Most CHAPLE patients show symptoms in early childhood, making timely trials even more important. Trials now focus not just on survival, but also on improving daily living and reducing hospital stays. International collaboration among hospitals and research labs is also rising. These trials offer hope to families waiting for better care options and signal more industry commitment to rare genetic disorders.
Increase in Global Awareness Programs
Awareness of CHAPLE syndrome is rising globally. Patient advocacy groups and research institutions are organizing educational campaigns to inform both healthcare providers and the public. Events like Rare Disease Day and online webinars are helping improve recognition of CHAPLE symptoms. Better awareness means doctors can diagnose the condition sooner, leading to quicker treatment. These programs also empower patients and families with the knowledge they need to manage care. As a result, more people are getting tested early, and clinical interest in CHAPLE syndrome continues to grow across different regions.
Use Cases
Treatment with Veopoz
Veopoz, approved in 2023, is the first targeted treatment for CHAPLE syndrome. It has helped reduce severe digestive symptoms like chronic diarrhea and abdominal pain. In clinical use, over 75% of patients showed improved protein levels in their blood. These patients also had fewer hospital visits for emergency care. Veopoz works by targeting the immune dysfunction caused by the CD55 deficiency. Regular use of this medication has shown to improve overall quality of life. This breakthrough has changed how CHAPLE syndrome is managed and has brought hope to patients and caregivers.
Supportive Care via Nutritional Therapy
CHAPLE syndrome often causes protein loss through the digestive system, leading to a condition called hypoalbuminemia. Many patients, especially children, face growth delays due to poor nutrition. More than 60% of diagnosed patients need high-protein diets and daily supplements. These nutritional strategies help manage symptoms and improve overall health. Specialized diet plans are developed with clinical nutritionists. Regular monitoring is required to track weight, energy levels, and protein levels. Without nutritional support, many patients risk severe complications. Diet remains a vital part of CHAPLE syndrome care.
Use of Genetic Testing for Diagnosis
Genetic testing plays a major role in diagnosing CHAPLE syndrome. The disorder is caused by mutations in the CD55 gene. Symptoms like chronic diarrhea, swelling, and poor growth often lead doctors to recommend DNA testing. Nearly 90% of confirmed CHAPLE cases are diagnosed through advanced genetic screening. Early diagnosis allows for quicker treatment and better symptom control. It also helps rule out other similar conditions. Testing is now more available in pediatric clinics and rare disease centers. This has made diagnosis faster and more accurate for affected families.
Home Infusion Services
Many CHAPLE patients now receive their medications through home infusion therapy. Veopoz is often given at home with the help of trained nurses. Around 40% of U.S. patients using Veopoz receive care this way. Home treatment reduces hospital trips and keeps the patient in a comfortable environment. It also ensures regular doses and fewer treatment delays. Families receive full support from home care teams to manage the process safely. This approach improves treatment adherence and lowers emotional stress. Home infusion is becoming a trusted option for long-term CHAPLE care.
Conclusion
The CHAPLE Syndrome Market is evolving steadily, driven by scientific breakthroughs, rising awareness, and the introduction of targeted therapies like Veopoz. With a projected CAGR of 4.4% through 2033, the market reflects growing recognition of this ultra-rare immune disorder and a stronger commitment to rare disease care. Key advancements in genetic testing, supportive nutrition, and home infusion services are enhancing patient outcomes and quality of life. As stakeholders continue investing in gene therapy, clinical trials, and collaborative care models, the CHAPLE syndrome market is set to expand with greater precision, accessibility, and innovation.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



