Table of Contents
Overview
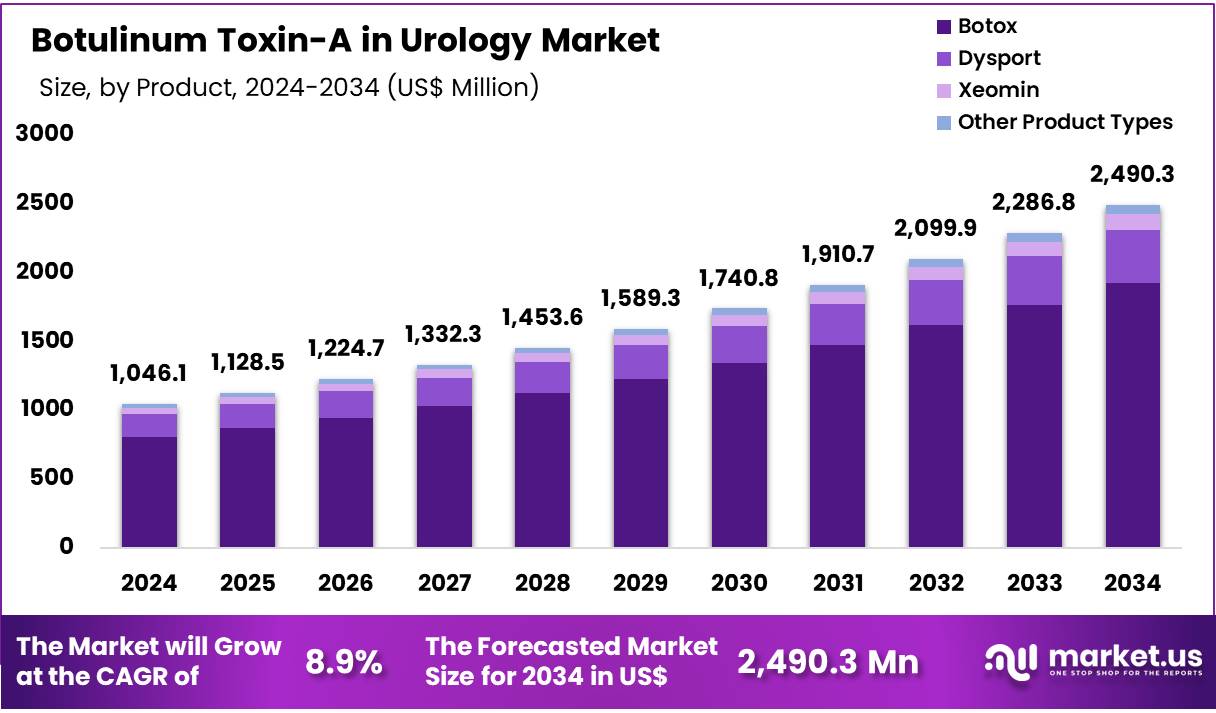
New York, NY – Nov 12, 2025 – Global Botulinum Toxin-A in Urology Market was valued at US$ 1,046.09 Million in 2024 and is expected to grow at a CAGR of 8.9% from 2024 to 2034. In 2024, North America led the market, achieving over 47.0% share with a revenue of US$ 491.29 Million.
The application of Botulinum Toxin-A (BoNT-A) in urology has emerged as a significant advancement in the management of various lower urinary tract dysfunctions. Initially recognized for its neuromuscular blocking properties, BoNT-A is now an established therapeutic option in conditions such as overactive bladder (OAB), neurogenic detrusor overactivity (NDO), and interstitial cystitis/bladder pain syndrome (IC/BPS).
The therapeutic action of BoNT-A is achieved through the inhibition of acetylcholine release at the neuromuscular junction, resulting in reduced detrusor muscle overactivity and bladder hypersensitivity. This targeted mechanism offers substantial relief from symptoms such as urinary urgency, frequency, and incontinence, thereby improving patients’ quality of life. Clinical studies have demonstrated its safety, efficacy, and durability of response, with repeat treatments showing consistent outcomes.
Regulatory approvals across multiple regions, including the U.S. FDA and EMA, have expanded its clinical use in both neurogenic and idiopathic cases. BoNT-A is administered through minimally invasive cystoscopic intradetrusor injections, offering a valuable alternative for patients unresponsive to conventional pharmacotherapy. The expanding role of BoNT-A in urology underscores its therapeutic versatility and growing relevance in modern clinical practice. Continued research and development are expected to further optimize its applications and explore new indications within functional urology.
Key Takeaways
- The Botulinum Toxin-A in Urology market generated US$ 1,046.09 Million in revenue and is projected to reach US$ 2,490.34 Million, registering a CAGR of 8.9%.
- Based on Product Type, the Botox products segment accounted for the highest revenue, representing a 77.2% market share.
- Based on Indication, the Overactive Bladder (OAB) segment dominated the market, capturing a 83.5% share.
- Based on End-User, the Hospitals segment led the market with a 67.4% share.
- Region-wise, North America remained the largest contributor, holding a 47.0% market share.
Regional Analysis
North America continues to hold a dominant position in the global Botulinum Toxin-A (BoNT-A) in Urology market, supported by advanced healthcare infrastructure, high healthcare spending, and rapid adoption of innovative treatment modalities. In 2024, the region accounted for 47.0% of the total market share, reflecting its strong leadership in clinical adoption and accessibility of BoNT-A therapies.
The United States remains the primary growth driver, attributed to the rising prevalence of urological disorders such as overactive bladder (OAB), neurogenic detrusor overactivity (NDO), and benign prostatic hyperplasia (BPH). As reported by the National Institutes of Health (NIH), OAB affects nearly 33 million Americans, indicating a substantial patient base for BoNT-A interventions. Favorable reimbursement frameworks and comprehensive insurance coverage in the U.S. further enhance treatment accessibility and encourage utilization of non-surgical therapeutic options.
Regulatory support and extensive clinical validation continue to reinforce the regional market expansion. The U.S. Food and Drug Administration (FDA) has approved BoNT-A for multiple urological indications, strengthening its clinical credibility among healthcare professionals.
Moreover, the regional inclination toward minimally invasive and long-lasting treatment solutions complements the benefits of BoNT-A therapy. With its growing demand for advanced urological care, North America is expected to maintain its market leadership, driving innovation and shaping the global BoNT-A in Urology landscape.
Emerging Trends
- Expanding Indications: The therapeutic use of Botulinum Toxin-A (BoNT-A) is extending beyond overactive bladder (OAB) and neurogenic detrusor overactivity (NDO) to conditions such as interstitial cystitis/bladder pain syndrome and chronic pelvic pain syndrome. Its neuromodulatory and anti-inflammatory properties support these expanding applications.
- Minimally Invasive Approaches: The growing preference for minimally invasive treatments has accelerated the adoption of BoNT-A injections. These procedures are favored for their shorter recovery times, lower complication risks, and reduced need for hospitalization compared to traditional surgical interventions.
- Long-Term Efficacy and Safety: Clinical studies confirm that BoNT-A maintains effectiveness with repeated administrations. Approximately 40% of patients experience symptom control lasting at least six months, while 30% report sustained benefits for over a year, demonstrating its durable therapeutic profile.
- Personalized Treatment Plans: Increasing focus is being placed on individualized treatment strategies. Adjusting BoNT-A dosage and injection sites according to patient-specific characteristics enhances therapeutic outcomes and minimizes potential adverse effects, reflecting the trend toward precision-based urological care.
Use Cases
- Idiopathic Overactive Bladder (OAB): In idiopathic OAB patients, BoNT-A significantly reduces urinary incontinence episodes. Systematic reviews show an average decrease of about 2.77 episodes per day compared to placebo, underscoring its strong clinical efficacy.
- Neurogenic Detrusor Overactivity (NDO): Among patients with spinal cord injury or multiple sclerosis, BoNT-A improves bladder storage function. Studies reveal an increase in maximum cystometric capacity from 182 mL to 313 mL within four weeks after treatment, indicating enhanced bladder control.
- Benign Prostatic Hyperplasia (BPH): BoNT-A injections into the prostate have demonstrated potential in managing BPH-related symptoms. Early clinical data show symptomatic improvement in 87% of treated individuals, compared to only 10% in control groups, highlighting its emerging therapeutic promise.
- Dysfunctional Voiding in Women: For women with refractory dysfunctional voiding, BoNT-A injections into the external urethral sphincter yield marked improvements. Patients exhibit reduced post-void residual volumes and better quality of life outcomes following treatment.
- Bladder Pain Syndrome/Interstitial Cystitis (BPS/IC): BoNT-A has shown meaningful clinical benefits in managing bladder pain and discomfort. Controlled studies report significant reductions in pain scores, suggesting its potential as a viable therapy for chronic bladder pain syndromes.
Frequently Asked Questions on Botulinum Toxin-A in Urology
- How does Botulinum Toxin-A work in urological treatments?
BoNT-A blocks the release of acetylcholine at the neuromuscular junction, reducing excessive muscle activity in the bladder wall. This results in improved bladder control, decreased urgency, and fewer episodes of incontinence. - What are the main indications for Botulinum Toxin-A in urology?
The primary indications include overactive bladder (OAB), neurogenic detrusor overactivity (NDO), and interstitial cystitis/bladder pain syndrome (IC/BPS). It is also explored for benign prostatic hyperplasia (BPH) and other urinary dysfunctions. - How is Botulinum Toxin-A administered for urological conditions?
BoNT-A is injected directly into the detrusor muscle of the bladder through a cystoscopic procedure. The treatment is minimally invasive and performed on an outpatient basis with minimal recovery time. - What are the benefits of Botulinum Toxin-A in urology?
BoNT-A provides long-lasting symptom relief, reduces urinary frequency and urgency, and enhances patient quality of life. It offers a non-surgical treatment option for patients unresponsive to conventional oral therapies. - Which product segment dominates the Botulinum Toxin-A in Urology market?
The Botox products segment leads the market, accounting for a 77.2% market share, driven by its strong brand recognition, proven efficacy, and wide regulatory approvals for urological applications. - Which indication contributes the most to market revenue?
The Overactive Bladder (OAB) segment holds the largest market share of 83.5%, owing to the high global prevalence of OAB and the established clinical success of BoNT-A in managing the condition effectively. - Which end-user segment leads the Botulinum Toxin-A in Urology market?
The Hospitals segment dominates with a 67.4% share, as most BoNT-A procedures are conducted in hospital settings equipped with advanced urology departments and specialized medical professionals. - Which region holds the largest share in the global market?
North America leads the global market with a 47.0% share, supported by advanced healthcare infrastructure, strong clinical adoption, favorable reimbursement policies, and a growing prevalence of urological disorders.
Conclusion
The global Botulinum Toxin-A (BoNT-A) in Urology market is experiencing strong growth, driven by rising prevalence of urinary disorders, expanding clinical indications, and increasing preference for minimally invasive treatments. With proven efficacy, long-term safety, and regulatory endorsements, BoNT-A has become a vital therapeutic option across neurogenic and idiopathic cases.
North America remains the market leader, supported by robust healthcare infrastructure and widespread clinical adoption. As research continues to explore new applications and personalized approaches, BoNT-A is expected to further strengthen its role in modern urological care, driving significant advancements in patient outcomes and market expansion globally.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



