Table of Contents
Overview
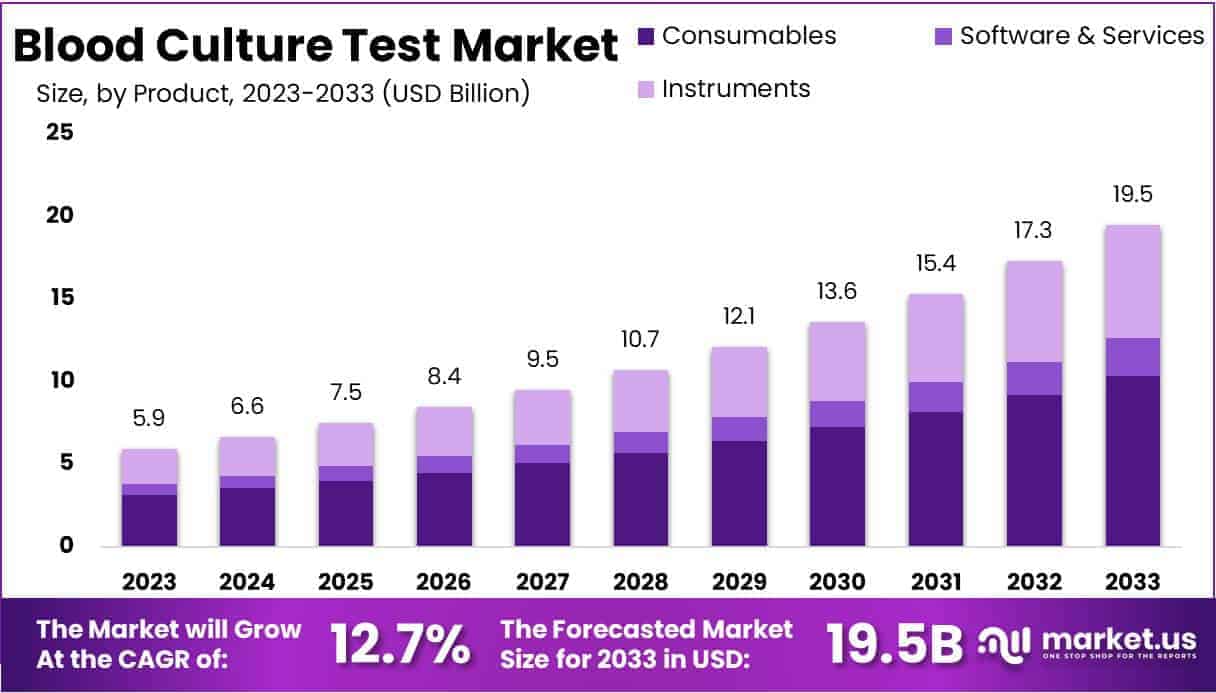
New York, NY – July 16, 2025: The Global Blood Culture Test Market is on a firm upward path. It was valued at USD 5.9 billion in 2023 and is projected to reach USD 19.5 billion by 2033, advancing at a 12.7 % CAGR. This momentum stems from the test’s status as an essential diagnostic. The World Health Organization places blood cultures on its recommended laboratory list, prompting health ministries to prioritise funding, training, and monitoring. When a technology earns an “essential” label, hospital budgets open up, policy barriers drop, and procurement volumes rise—all ingredients for sustained demand growth.
A sharp rise in bloodstream infections reinforces that demand. Global surveillance systems, from the U.S. CDC to the European ECDC, report higher sepsis and catheter‑related infection rates. Clinicians depend on blood cultures to detect pathogens quickly, guide targeted therapy, and cut mortality. Faster, more accurate diagnostics also reduce unnecessary broad‑spectrum antibiotic use, a key step in curbing antimicrobial resistance. In intensive‑care and emergency settings, every hour saved on pathogen identification lifts survival odds and shortens length of stay.
Government action is amplifying these clinical drivers. Many countries now run nationwide infection‑tracking programmes that require routine blood culture reporting. Hospitals must submit isolate data and susceptibility profiles, creating a feedback loop that improves local treatment guidelines. Reimbursement rules have moved in tandem; several public insurers now link payment to compliance with sepsis bundles that include timely blood cultures. Such policies turn best practice into standard practice, further widening the installed base of automated culture instruments and consumables.
Cost remains a restraint in low‑ and middle‑income regions, yet progress is evident. Global health agencies, philanthropic donors, and pooled procurement groups negotiate discounted prices and promote local bottle manufacturing. Simplified protocols also limit waste and training overhead. These efforts enable smaller hospitals to adopt dependable testing without straining budgets. Overall, a convergence of clinical urgency, policy endorsement, and technological innovation positions blood culture testing as a critical growth segment through 2033, with robust opportunities for both established and emerging suppliers.
Key Takeaways
- In 2023, the Blood Culture Test market earned USD 5.9 billion and is projected to reach USD 19.1 billion by 2033.
- The consumables segment dominated product sales, capturing 53.2% of the total market revenue due to frequent usage and recurring demand in testing.
- Conventional techniques led the market by technique type, accounting for a 59.2% share, showing strong continued use in diagnostic labs.
- Culture-based technology emerged as the most used testing method, contributing 42.5% of the market, owing to its proven reliability in detecting bloodstream infections.
- Hospital laboratories took the lead among end-users in 2023, holding a 62.8% share, as they are the primary sites for critical infection testing.
- North America held the highest regional share at 38.2%, driven by advanced healthcare systems and increased investment in infectious disease diagnostics.
Segmentation Analysis
In 2023, consumables led the blood culture tests market, securing 53.2 % of total revenue. Demand is rising because more blood cultures are ordered, infectious diseases remain prevalent, and awareness keeps growing. Clinicians value early detection, so they use more bottles, media, and safety sets. Technology boosts this trend. New designs reduce contamination, cut waste, and speed workflows. For example, Kurin launched a push‑button needle system in February 2022 that shields staff and patients. Such advances should keep the consumables segment on a fast CAGR path.
Conventional techniques still dominate processing. In 2023 they accounted for 59.2 % of global volume. Hospitals and basic labs favour glass bottles and manual incubation because the tools are proven and cheap. The method is familiar and flexible. These traits sustain use in both mature and resource‑limited settings. Yet research groups are upgrading the workflow. A December 2022 study by Great Ormond Street Institute and the Wyss Institute re‑engineered pediatric sepsis protocols. The revised approach cut time to pathogen detection. Such pilot wins will drive wider change.
Hospital laboratories remain the chief end users. They held 62.8 % of market share last year. High admission rates and stubborn hospital‑acquired infections fuel this lead. The World Health Organization noted in 2022 that seven percent of patients in rich systems acquire an infection during a stay. The rate jumps to fifteen percent in low‑ and middle‑income settings. Rapid blood cultures guide therapy, so clinicians test early and often. In many emerging regions, hospitals house the only incubators. Their role is therefore even more critical.
By Product
- Consumables
- Software & Services
- Instruments
By Technique
- Conventional
- Automated
By Technology
- Culture-based Technology
- Molecular Technology
- PCR
- Microarray
- PNA-FISH
- Proteomic Technology
By Application
- Bacterial Infections
- Fungal Infections
- Mycobacterial Infections
End User
- Hospital Laboratories
- Reference Laboratories
- Academic Research Laboratories
- Other Laboratories
Regional Analysis
In 2023, North America held the largest share of the global blood culture test market, accounting for 38.2% of total revenue. This dominance is supported by a strong clinical diagnostics sector and the presence of leading industry players. Government-funded programs and a supportive reimbursement system help ensure broad access to advanced testing technologies. The region also benefits from ongoing awareness campaigns that highlight the importance of timely blood culture tests. These efforts are improving early infection detection and boosting market demand across hospitals and diagnostic centers.
Innovation plays a key role in North America’s market leadership. In February 2023, the FDA approved the Selux NGP System, developed by Selux Diagnostics, Inc. and BARDA. This system enables faster testing by processing samples directly from positive blood cultures. It reduces test time from 36 hours to just 16 hours after whole blood collection. Such technological advances support quicker treatment decisions, better patient outcomes, and overall efficiency in infection management. These factors continue to drive market growth in the region.
Key Players Analysis
The global blood culture test market has a vibrant competitive landscape driven by a few strong brands. BioMérieux, BD, T2 Biosystems, and Beckman Coulter lead the field with broad portfolios and global distribution. Each firm targets niche needs through innovation and rapid diagnostic platforms. New automated instruments and faster incubation systems create clear points of differentiation. Firms invest in digital integrations and data analytics to improve lab workflow. This focus on speed, accuracy, and usability helps gain share among hospital laboratories worldwide.
Strategic partnerships amplify this competitive push. BD’s alliance with Magnolia Medical Technologies expands its specimen‑collection solutions and deepens customer trust. Similar collaborations give companies early access to novel detection methods and flexible distribution channels. Regulatory approvals, such as the FDA’s clearance of new rapid panels, create barriers for late entrants and build credibility for incumbents. Meanwhile, rising antimicrobial resistance triggers higher R\&D spending on molecular assays and AI‑driven reporting. These factors foster intense rivalry yet also enlarge the overall revenue pool.
- Abbott Laboratories
- Accelerate Diagnostics
- Alere Inc. (now part of Abbott)
- Beckman Coulter (a subsidiary of Danaher Corporation)
- Becton, Dickinson and Company (BD)
- bioMérieux
- Bio-Rad Laboratories
- Bruker Corporation
- Cepheid (a Danaher company)
- COPAN Diagnostics, Inc.
- Luminex Corporation
- Roche Diagnostics
- Siemens Healthineers AG
Emerging Trends
Direct Testing from Culture Bottles
Advanced analyzers now bypass many legacy steps. Instead of sub‑culturing on plates, the instrument injects reagents straight into the bottle. It then amplifies pathogen DNA and reads resistance genes in one run. Results arrive within hours, not days. Consequently, the lab saves labor and consumables. Clinicians like the speed because it trims empirical therapy. Patients benefit from targeted antibiotics sooner, which reduces side effects. In addition, faster clearance shortens length of stay. Infection‑control teams use the quick data to track outbreaks in real time. Altogether, bottle‑to‑result systems are reshaping daily microbiology practice.
Artificial Intelligence Is Improving Workflow
Hospitals mine electronic records for hidden infection clues. Machine‑learning tools flag abnormal vitals, lab trends, and device use. The algorithm then suggests a blood culture at the right moment. This targeted approach cuts unnecessary draws and lowers contamination. Doctors trust the alert because it explains its reasoning in plain language. When combined with rapid diagnostics, AI shortens the decision loop even further. Moreover, predictive dashboards help managers allocate staff to high‑risk wards. Early warning also prompts timely isolation, which curbs spread. As datasets grow, these models learn fast and keep refining their accuracy.
Stricter Quality Standards in Hospitals
Health authorities demand cleaner blood culture draws. New guidelines limit skin punctures per patient and specify antiseptic contact time. Labs must track contamination rates and publish monthly dashboards. When metrics slip, teams run root‑cause drills and retrain staff. Lower false‑positive counts save antibiotics and prevent unnecessary line removals. Insurance providers reward facilities that hit quality benchmarks with better reimbursement. Patients notice fewer needle sticks and shorter stays. Ultimately, high standards build public trust in hospital safety. Compliance also prepares institutions for surprise accreditation audits and strengthens overall infection‑control programs.
More Focus on Infection Control and Public Health
National surveillance networks now collect anonymized culture results every day. Analysts map hotspots for drug‑resistant bacteria and send alerts to local clinics. Policymakers then adjust antibiotic‑stewardship campaigns by region. During outbreaks, real‑time data guides rapid stockpiling of critical drugs. Global agencies aggregate trends to spot emerging threats before they cross borders. Hospitals that share timely numbers gain access to research grants and training. Moreover, transparent reporting fosters collaboration between human and veterinary health sectors. Ultimately, broad data sharing turns individual blood cultures into a powerful tool for safeguarding community health.
Use Cases
Child and Maternal Health
In many developing regions, newborns and pregnant women face high infection risks. Blood‑culture testing offers the first clear picture of dangerous bacteria. Midwives or community nurses take tiny blood volumes using sterile kits. Portable incubators or rapid cartridges start analysis right at the clinic. Early detection means antibiotics reach babies or mothers before sepsis spreads. Timely treatment cuts deaths, prevents disabilities, and protects future pregnancies. Validated culture data also guides national vaccine programs against major pathogens. Reliable numbers attract funding from donors focused on maternal and child survival.
Rural and Remote Healthcare Settings
Rural clinics often sit hours away from full diagnostic laboratories. During outbreaks, waiting for central results can be deadly. Hand‑held blood‑culture devices now run on battery power and cellular networks. Workers collect a sample, load a cartridge, and start incubation on site. Positive signals send alerts to doctors via simple text messages. Treatment starts the same day instead of after risky travel. Early action limits disease spread within tight rural communities. Data uploaded to national dashboards helps officials map and stop outbreaks. These portable tools bring urban‑level diagnostics to the edge of care.
Predictive Healthcare Tools
Hospitals with advanced analytics are moving from reactive to predictive infection control. Machine‑learning models scan vitals, lab trends, and device data in real time. When risk scores cross a threshold, the system suggests drawing a blood culture. Clinicians receive the alert directly in their electronic medical‑record inbox. Early sampling often catches pathogens before fevers spike or organs suffer. Quick, targeted therapy reduces septic‑shock cases and readmissions. Feedback loops retrain the model, making each prediction cycle more accurate. Data‑driven timing saves supplies and protects patients from unnecessary sticks.
Managing Medical Supplies
Pandemics, strikes, or shipping delays can suddenly shrink hospital supply shelves. When blood‑culture media run low, stewardship committees step in. They rank patients by infection risk, ensuring the highest‑value tests are done. Pharmacies receive daily inventories linked to expected culture demand. Digital dashboards show staff which wards still have testing kits available. This transparency prevents hoarding and wastage in low‑risk units. Prioritized testing keeps critically ill patients safe even during shortages. Clear rules also reassure clinicians that rationing is science‑based, not arbitrary.
Conclusion
The blood culture test market is growing fast due to its vital role in identifying infections early and improving treatment. Hospitals, labs, and governments are investing in better tools and faster testing methods. New technologies and AI are making diagnostics quicker and more accurate, helping doctors act faster and save lives. Supportive policies, training, and public health programs are also pushing adoption across regions. Even in low-resource areas, new devices and funding are making these tests more available. As awareness and demand rise, more suppliers are entering the space with advanced solutions. Blood culture testing is now a key part of modern healthcare and infection control worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



