Table of Contents
Introduction
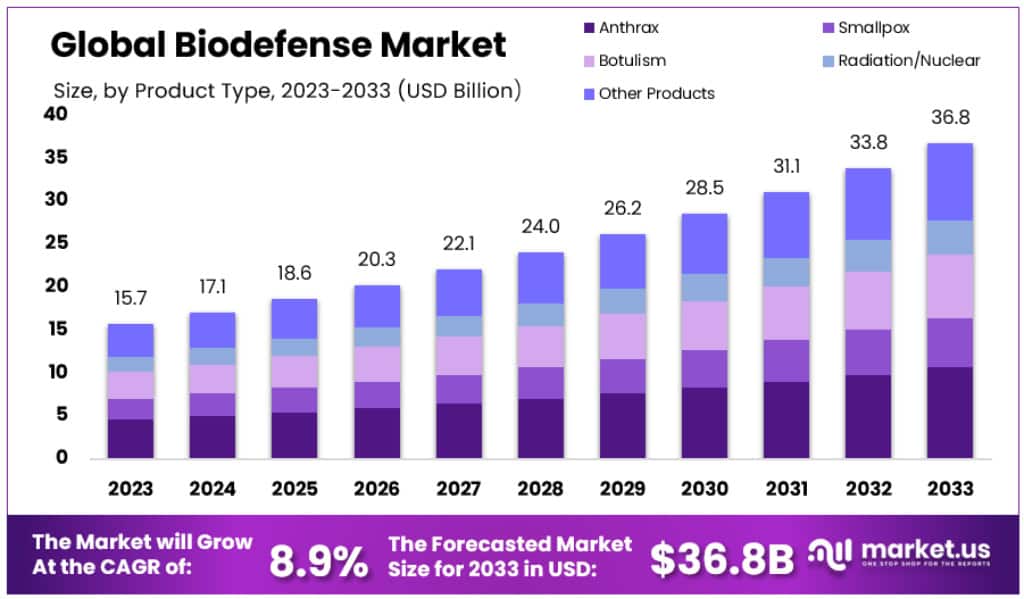
Global Biodefense Market size is expected to be worth around USD 36.8 billion by 2032, from USD 15.7 billion in 2023, growing at a CAGR of 8.9% during the forecast period from 2024 to 2033.
Biodefense encompasses strategic measures to prepare for, respond to, and counter bioterrorism a deliberate act involving the release of harmful biological agents such as viruses and bacteria that pose severe threats to public health, agriculture, and the environment. This field integrates medical responses like vaccination and therapeutic interventions to shield populations from biological hazards.
Significant funding from both governmental and private sectors, alongside strategic product acquisitions, significantly bolsters the biodefense market. Initiatives such as Project BioShield are pivotal, offering countermeasures against biological threats like anthrax and smallpox, as well as chemical, nuclear, and radiological agents. The establishment of various funding programs aims to enhance research and development efforts and advance the next generation of biodefense strategies.
Technological breakthroughs in genetic engineering and biotechnology over recent decades have simplified the modification of lethal pathogens, which can be engineered for mass disruption. The ease of access to these organisms highlights the critical need for robust biodefense mechanisms globally. Historical instances of bioterrorism using agents like anthrax and botulism underscore the significant economic and health repercussions of these threats.
Key Takeaways
- Market Size: The Global Biodefense Market size is expected to be worth around USD 36.8 billion by 2032, from USD 15.7 billion in 2023.
- Market Growth: The market growing at a CAGR of 8.9% during the forecast period from 2024 to 2033.
- Product Analysis: The market for biodefense was dominated by the anthrax segment, which held the largest revenue share with 29% in 2023
- Application Analysis: Due to terrorist organizations’ increasing biological threats to civilians, the revenue CAGR for the civilian segment will be rapid.
- End-Use Analysis: the Hospitals & Clinics segment emerged as the dominant force, commanding approximately 60% of the market share.
- Regional Analysis: North America Leading the Market: In 2023, North America, especially the United States, dominated the biodefense market, holding about 80% share, valued at $12.6 million.
Biodefense Statistics
- Personal Protective Equipment (PPE) Surge Capacity: The U.S. Department of Health and Human Services (HHS) maintains a minimum 90-day PPE surge capability to support control of pandemics and outbreaks.
- COVID-19 Vaccine Distribution: More than 284 million COVID-19 vaccine doses have been administered through the Federal Retail Pharmacy Program.
- Vaccine Development Funding: BARDA has awarded $6.8 million to develop alternative vaccine delivery methods, including microneedle skin patches and oral vaccines.
- Diagnostic Test Volume: BARDA-supported COVID-19 tests account for more than one-fifth of the total U.S. molecular lab test volume.
- Biodefense Exercises: From 2009-2019, federal agencies conducted 74 interagency exercises to prepare for biological incidents.
- Clinical Trials Infrastructure: HHS aims to administer candidate countermeasures within 14 days from the identification of a viable countermeasure.
- Funding for Vaccine Delivery Innovations: 10 small biotechnology companies and universities are funded under BARDA’s Beyond the Needle program.
- International Health Security Support: The U.S. supports 50 countries to enhance global health security initiatives.
- Pharmacy Involvement in Vaccine Distribution: 21 pharmacy organizations participate in distributing COVID-19 vaccines through a partnership with CDC.
- Funding for COVID-19 Diagnostic Tests: In 2020, HHS allocated $29 million for developing and distributing COVID-19 diagnostic tests.
- Influenza Vaccine Development: NIH has multiple preclinical universal influenza vaccine candidates in advanced stages.
- Antiviral Drug Development: NIH has established nine Antiviral Drug Discovery Centers to address pathogens of pandemic concern.
- Dissolution of Biodefense Committees: The Biodefense Coordination Team and Biodefense Steering Committee were dissolved in 2022 under the new National Biodefense Strategy.
- Launch of the New Biodefense Strategy: The 2022 National Biodefense Strategy was launched by the White House, focusing on integrated biodefense measures.
- Biodefense Research Funding: The U.S. spends over $100 million annually on biodefense-related research and development.
- Biodefense Incident Response Time: The U.S. aims to respond to biodefense incidents within 24 to 48 hours of identification.
Biodefense Product Analysis
- Anthrax: The primary biodefense against anthrax involves the BioThrax vaccine, which is the only FDA-approved vaccine for pre-exposure prophylaxis of anthrax disease. It is used extensively by the U.S. Department of Defense, particularly to protect military personnel, under a contract worth up to $235.8 million. This contract includes a ten-year plan to supply these vaccines, demonstrating significant governmental commitment to anthrax preparedness.
- Botulism: The biodefense strategy against botulism primarily involves stockpiling antitoxins and maintaining a strong surveillance system to quickly detect and respond to incidents. Botulism antitoxin is critical due to the rapid progression of the disease’s symptoms, and timely distribution can be lifesaving.
- Smallpox: The United States has maintained a significant stockpile of smallpox vaccine, known as ACAM2000, which is available for use in the event of an outbreak. The vaccine is part of a broader strategy to be prepared against this eradicated virus, which could potentially be used as a bioweapon.
- Nuclear/Radiation: Preparedness for nuclear or radiation emergencies includes a range of countermeasures, such as Potassium Iodide (KI) to protect the thyroid gland from radioactive iodine, and Prussian blue, which is used to treat cesium or thallium contamination. These countermeasures are crucial components of emergency medical kits distributed in scenarios of radiological or nuclear exposure.
- Other Products: The biodefense market also includes broad-spectrum antivirals and antibiotics, which are essential for dealing with lesser-known or emerging biological threats. These are part of strategic national stockpiles to ensure responsiveness to a variety of biological threats, including emerging infectious diseases and potential bioterrorism agents not covered individually by more specific products.
Emerging Trends
- Advancement in Biosurveillance Technologies: The Department of Homeland Security is enhancing biodefense capabilities through advanced biosurveillance technologies. These technologies are designed to provide early warnings and monitor biological threats more efficiently, aiming to reduce response times significantly.
- Integration Across Military and Civilian Sectors: The U.S. Department of Defense emphasizes the importance of a total force approach in biodefense, incorporating all branches of the military and civilian sectors. This integration ensures a unified response to biological threats, enhancing readiness and resilience across all levels.
- Rapid Development and Deployment of Medical Countermeasures (MCMs): Emphasis is being placed on accelerating the production and distribution of vaccines and therapeutics. This includes optimizing manufacturing processes and forming robust partnerships to ensure quick deployment during crises, essential for responding to fast-evolving biological threats.
- Holistic “One Health” Approach: The U.S. government is adopting a “One Health” strategy that interweaves human, animal, plant, and environmental health efforts. This approach helps in creating a comprehensive response system that is effective against a wide array of biological threats.
- Innovative Use of Genomic and Wearable Technologies: There is a significant push towards employing genomic sequencing and wearable technologies for early detection and real-time monitoring of disease outbreaks. These technologies play a crucial role in the rapid identification and containment of pathogens.
- Enhanced Interagency and International Collaboration: Biodefense strategies now emphasize greater collaboration across different government sectors and with international partners. This collaborative effort is crucial for developing and implementing effective global biodefense strategies, highlighting the need for synchronized actions to combat biological threats efficiently.
Use Cases
- Rapid Response to Biological Threats: The National Biosurveillance Integration Center (NBIC) effectively identifies and tracks biological events such as avian flu, integrating data from diverse sources to provide early warnings and enhance situational awareness.
- Advanced Medical Countermeasures: The U.S. has implemented robust systems to quickly develop and distribute vaccines and therapeutics in response to biological threats, with streamlined manufacturing techniques and rapid regulatory pathways to ensure timely availability.
- Enhanced Biosurveillance Capabilities: The Department of Homeland Security has developed advanced biosurveillance programs like BioWatch and the National Biosurveillance Integration Center to improve early detection and response to biological incidents, ensuring comprehensive threat analysis and rapid information sharing.
- Integration of Biodefense Strategy: The Apollo Program for Biodefense aims to create a proactive and unified national response system capable of rapidly detecting and responding to any biological threat, emphasizing rapid diagnostic distribution and vaccine development.
- Interagency Coordination for Comprehensive Preparedness: The updated National Biodefense Strategy outlines a coordinated approach across multiple federal agencies and departments to ensure a unified and effective response to biological threats, including the establishment of clear communication and operational strategies.
- Global Health Security Enhancement: The National Biodefense Strategy emphasizes international collaboration to enhance global health security, ensuring that the U.S. and its partners are prepared to prevent, detect, and respond to biological threats effectively.
Conclusion
The biodefense market is poised for significant expansion, driven by increasing biological threats and technological advances in biosurveillance and medical countermeasures. With an expected market size of USD 36.8 billion by 2032, strategic investments in vaccine development, diagnostic tools, and international collaboration underscore a proactive approach to global biodefense. The integration of military and civilian efforts enhances readiness against bioterrorism, while emerging technologies facilitate rapid response capabilities. Collectively, these developments ensure a resilient infrastructure to safeguard public health and security against potential biological threats.


