Table of Contents
Introduction
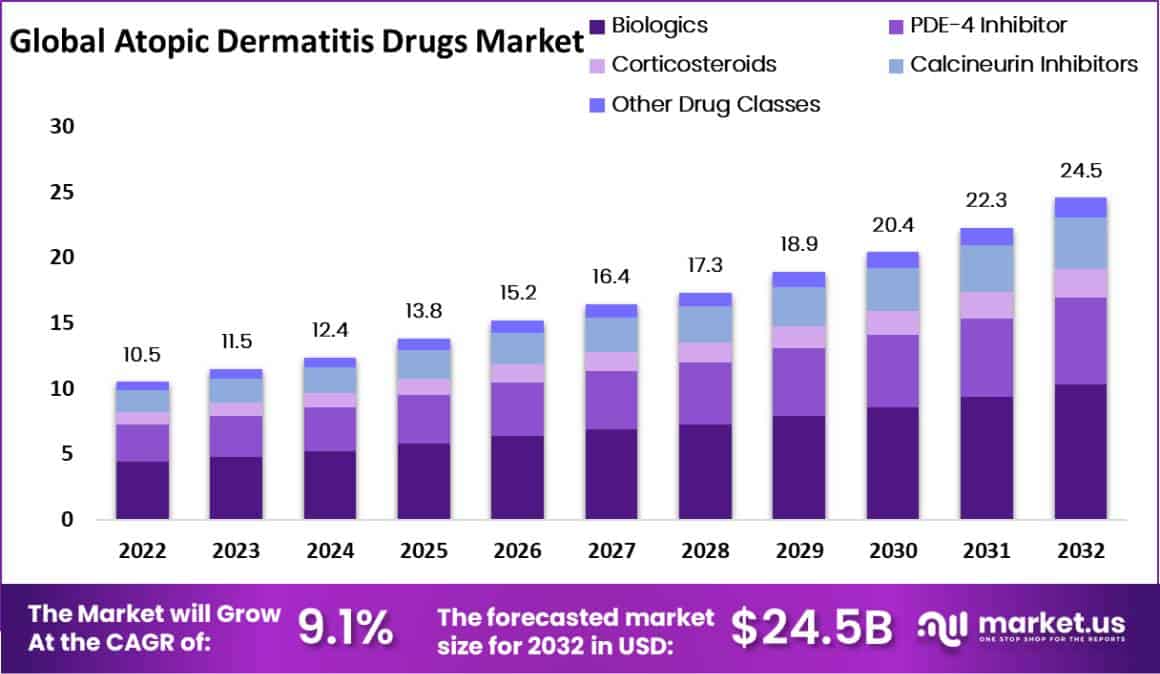
The global Atopic Dermatitis Drugs Market is projected to grow from USD 10.5 billion in 2023 to around USD 24.5 billion by 2033, with a compound annual growth rate (CAGR) of 9.1% during the forecast period from 2024 to 2033. This growth is primarily driven by the increasing prevalence of atopic dermatitis (AD), significant advancements in therapeutic options, and the profound economic and quality of life impacts associated with the disease.
AD affects a substantial demographic, including up to 25% of children and 2-3% of adults globally. The heightened awareness and prevalence have catalyzed the development of effective management strategies and drugs, which are essential growth drivers in this market sector. As awareness increases, so does the demand for innovative and effective treatments, underscoring the importance of sustained research and development.
Innovations in treatment options such as biologics and Janus kinase (JAK) inhibitors have revolutionized the management of moderate to severe AD. These advanced therapeutics target specific pathways involved in inflammation and itching, providing relief and improving the quality of life for patients. The ongoing development and approval of these drugs are expected to continue bolstering market expansion, addressing the needs of a growing patient population seeking effective AD management solutions.
The economic burden and quality of life deterioration associated with AD are significant. Patients frequently incur higher healthcare costs, including increased doctor visits and hospitalizations, particularly those with severe conditions. The direct costs, alongside indirect expenses such as lost work productivity, emphasize the urgent need for effective treatments. This economic impact motivates continuous research and enhances the market’s growth potential as new therapies emerge.
Recent developments further illustrate the sector’s dynamism. In September 2024, Eli Lilly received FDA approval for EBGLYSS (lebrikizumab-lbkz) for treating moderate-to-severe AD in adults and children aged 12 and older. This approval was based on the drug’s efficacy demonstrated in Phase 3 trials, where it significantly cleared skin and relieved itching symptoms. Earlier, in June 2023, Sanofi reported promising Phase 2b trial results for amlitelimab, showing a notable reduction in Eczema Area and Severity Index scores, positioning it as a potential first-in-class option for AD treatment. These milestones underscore the sector’s vibrant growth trajectory and the continuous improvement in therapeutic offerings.
Key Takeaways
- The Atopic Dermatitis Drugs Market is set to grow rapidly from 2022 to 2032, with an annual growth rate of 9.1%.
- Worldwide, Atopic Dermatitis impacts 10.2% to 17.1% of adults and 0.96% to 22.6% of children.
- In 2022, biologics held a 42% market share, supported by government initiatives, a strong pipeline, and effectiveness in severe cases.
- Injectable drugs represented 46% of the market, increasingly used especially among pediatric patients.
- Hospital pharmacies are the leading distribution channel, boosted by new drug approvals and governmental backing.
- Market growth is driven by the rising global prevalence of atopic dermatitis and higher demand for biologic treatments.
- Potential side effects from long-term use of strong topical steroids pose a market challenge.
- New opportunities are emerging from innovative drugs in the pipeline and favorable reimbursement policies in developing nations.
- North America leads the market with a 52% revenue share, influenced by beneficial reimbursement setups and increased hand eczema cases post-COVID-19.
Atopic Dermatitis Drugs Statistics
Prevalence and Impact
- Atopic dermatitis (AD) affects 20% of children globally.
- Around 10-20% of children with AD continue to have symptoms into adolescence.
- Adolescent AD prevalence ranges from 5% to 20%, varying by region.
- In Asia-Pacific, AD affects 10.1% of children aged 6–7 years and 5.3% of adolescents aged 13–14 years.
- The prevalence of AD among Philippine children under 18 is 12.7%, but only 2% in adults.
- In Singapore and Malaysia, AD prevalence in young adults is 13.5%, with 40.5% experiencing moderate to severe symptoms.
- Severe AD affects approximately 1.2% of children and 0.7% of adolescents in Asia-Pacific.
- Adults in Singapore showed an 18% rate of anxiety due to AD; 5% experienced depression symptoms.
- In Taiwan, 89.2% of working individuals with AD report reduced productivity or absences.
- AD patients are 44% more likely to consider suicide compared to those without AD.
Clinical Trials and Treatments
- Upadacitinib trials lasted 76 weeks, assessing its efficacy in adolescents across three studies: Measure Up 1, 2, and AD Up.
- The studies included sample sizes of 179, 180, and 183 adolescents, respectively.
- Over 70% of adolescents achieved a 75% improvement in Eczema Area Severity Index (EASI-75) by week 16 using upadacitinib.
- Upadacitinib’s effectiveness was maintained or improved through week 76.
- The treatment showed serious infections in 5 or fewer participants across all study arms.
- The most common side effects included acne, mostly mild and rarely leading to discontinuation.
- Two instances of rhabdomyolysis were reported, related to increased physical activity, with one case requiring discontinuation.
- Eczema Herpeticum was mostly mild or moderate, with only one severe case leading to discontinuation.
Current Medications and Developments
- Dupilumab, targeting IL-4 and IL-13, was FDA approved in 2016 and by the EMA in 2017 for moderate to severe AD.
- Baricitinib, a JAK1/JAK2 inhibitor, received European approval in 2020 for adults with moderate to severe AD.
- Tralokinumab, an anti-IL-13 antibody, was approved by the EMA in 2021.
- Over 70 new compounds are currently under development for AD treatment.
- Early Phase trials of bispecific antibodies suggest potential for reducing inflammation and pruritus.
- Rademikibart, an IL-4Rα inhibitor, showed a 63% reduction in EASI at 16 weeks.
- Eblasakimab, targeting IL-13Rα1, achieved a 73% improvement in EASI scores in Phase 2b trials.
- Nemolizumab, an IL-31Rα inhibitor, led to a 63.1% reduction in itch scores within 12 weeks.
- Amlitelimab, inhibiting the OX40-OX40L pathway, reached EASI-75 in 59% of participants in a Phase 2a study.
Medication Adherence and Economic Impact
- Japanese patients show low adherence to medication: 66.3% for oral and 75.5% for topical treatments.
- Adherence to dupilumab self-injections in Japan is just 59.4%.
- In Taiwan, fears of corticosteroids significantly affect treatment adherence.
- AD treatment costs in India account for about 25% of a patient’s annual earnings.
- In Taiwan, the annual cost related to work productivity loss per AD patient ranges from USD 6,346 to USD 9,310.
- Tapinarof, a natural AhR agonist, met its primary endpoint in 53% of Phase IIb trial participants.
- Tezepelumab allowed 64.7% of patients to reach the EASI50 endpoint in a Phase IIa study.
- Etokimab showed an EASI75 result in 33% of patients in a proof-of-concept study, lasting up to 140 days post-dose.
- Upadacitinib significantly met the primary endpoint of IGA 0/1 at week 16 in Phase III trials.
- Abrocitinib displayed significant efficacy in Phase III trials, especially in early itch response after 2 weeks.
- FB-401 demonstrated significant disease improvement in 60% of adult and 90% of pediatric patients in early Phase I/IIa studies.
- Niclosamide ATx201 cream showed significant histological and transcriptional improvements in skin condition during Phase II trials.
Emerging Trends
- Biologic Therapies: Recent advancements in atopic dermatitis treatments have introduced biologic drugs, which are engineered to target specific immune responses. Unlike conventional treatments that offer broader effects, biologics focus on disrupting cytokine signaling, a critical element in the inflammatory process of atopic dermatitis. This method promises more precise and effective treatment by directly interfering with the pathways that contribute to the disease’s symptoms.
- JAK Inhibitors: The advent of Janus kinase (JAK) inhibitors marks a significant evolution in managing atopic dermatitis. These drugs specifically inhibit enzymes that play a crucial role in the inflammation pathway, offering a more targeted approach to symptom management. The approval and introduction of JAK inhibitors have opened new avenues for effectively controlling the disease with fewer side effects compared to traditional therapies.
- Personalized Medicine: There is an increasing shift towards personalized medicine in treating atopic dermatitis, driven by a deeper understanding of the disease’s molecular basis. Treatment plans are increasingly being customized based on individual genetic profiles, environmental factors, and unique immune responses. This trend is supported by technological advances that allow for more precise identification of the molecular mechanisms underlying different patients’ conditions, leading to more effective and tailored treatments.
- Topical and Systemic Combination Therapies: For severe cases of atopic dermatitis, there is a growing trend towards using systemic treatments in conjunction with topical agents. This combination approach leverages the strengths of both treatment modalities—systemic immunomodulators provide broad immune control, while topical treatments address local skin symptoms. This strategy is becoming essential for comprehensive disease management, particularly in patients with extensive or resistant atopic dermatitis.
- Improved Drug Delivery Systems: Innovation in drug delivery systems continues to enhance the treatment of atopic dermatitis. Advances include better topical formulations and targeted delivery technologies that improve the drug’s efficacy and ease of use. These innovations are crucial for enhancing patient adherence to treatment regimens and ultimately improving outcomes. Improved delivery systems ensure that medications are more effectively absorbed and utilized, minimizing waste and maximizing therapeutic effects.
Use Cases
- Severe Disease Management: In severe cases of atopic dermatitis, where traditional topical treatments are insufficient, systemic medications such as biologics and JAK inhibitors become crucial. These treatments are designed to significantly lessen symptoms and enhance the patient’s quality of life. By targeting underlying causes of inflammation, these drugs offer a powerful solution for those who suffer intensely from this condition. This approach not only alleviates discomfort but also reduces the frequency and severity of flare-ups, making it a vital component of treatment for severe atopic dermatitis.
- Maintenance Therapy: To manage atopic dermatitis effectively, the focus is shifting towards maintenance therapy using newer pharmacological agents. These drugs are not just for treating symptoms as they arise but are integral in preventing future flare-ups. This proactive strategy involves regular medication to maintain skin health and prevent the recurrence of severe symptoms. It’s a transformative approach that prioritizes continuous management over reactive treatment, marking a significant advancement in how this chronic condition is treated.
- Pediatric Applications: Recognizing the specific needs of children with atopic dermatitis, pharmaceutical companies are developing special formulations and dosages tailored for young patients. These pediatric treatments are designed to be both effective and gentle, minimizing side effects while addressing the dermatological needs of children. This focus on pediatric care is essential, as children constitute a large segment of the patient population for atopic dermatitis. By providing safer and more tolerable options, these advancements significantly improve the therapeutic outcomes for affected children.
- Combination Therapy for Acute Flares: When dealing with acute flare-ups of atopic dermatitis, employing a combination of topical and systemic treatments is often necessary. This integrated treatment strategy ensures rapid and effective control of symptoms. By using multiple therapeutic approaches simultaneously, medical practitioners can more effectively manage these critical situations. This method underscores the importance of a versatile and responsive treatment regimen to address the complexities of atopic dermatitis during intense episodes.
- Lifestyle Integration: Modern treatment strategies for atopic dermatitis increasingly emphasize the integration of lifestyle and environmental modifications along with pharmacological interventions. Patients are advised on how to avoid known triggers and incorporate suitable skincare routines that support the effectiveness of their drug treatments. This holistic approach not only helps manage the disease more effectively but also enhances the overall treatment experience by aligning daily habits with medical advice, fostering a comprehensive management strategy that extends beyond medication alone.
Conclusion
In conclusion, the global market for atopic dermatitis drugs is on a robust growth trajectory, expected to reach approximately USD 24.5 billion by 2033, driven by the increasing prevalence of the condition and significant innovations in treatment options. The introduction of biologics and JAK inhibitors has been pivotal, offering targeted relief for moderate to severe cases and enhancing the quality of life for patients. These developments, coupled with a growing awareness of the economic and lifestyle impacts of atopic dermatitis, continue to fuel research and demand for more effective treatments. As the market evolves, the focus on personalized medicine and improved drug delivery systems is likely to offer new opportunities and drive further advancements in managing this challenging skin condition.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



