Table of Contents
Overview
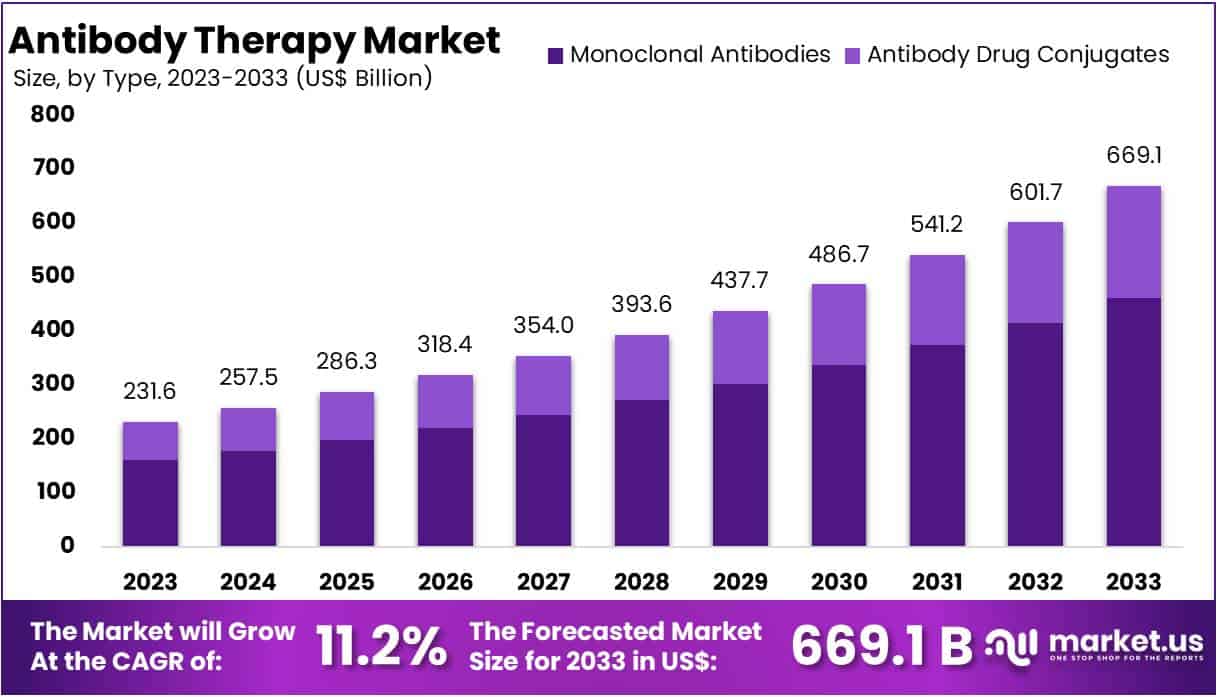
New York, NY – July 14, 2025: The Global Antibody Therapy Market is entering a rapid growth phase. It was valued at about US$ 231.6 billion in 2023 and is projected to reach roughly US$ 669.1 billion by 2033. This reflects a compound annual growth rate of 11.2 % from 2024 to 2033. Demand is driven by the therapy’s wide application in oncology, autoimmune disease, and infectious‑disease care. Physicians favour antibodies because they bind directly to specific targets, sparing healthy tissue and reducing side effects. As payers seek treatments that improve outcomes while controlling costs, precision biologics are moving to the centre of many treatment guidelines. This baseline sets the stage for continued industry expansion.
The need for precise treatment is rising as chronic and complex illnesses become more common worldwide. The World Health Organization warns that cancer, autoimmune disorders, and stubborn viral infections are all increasing. Traditional small‑molecule drugs often struggle to keep pace. Antibody therapies meet the challenge by delivering targeted action with fewer side effects, which lifts adherence and quality of life. In oncology, checkpoint inhibitors and bispecific antibodies are widening survival curves. In autoimmune care, newer monoclonal antibodies reduce flares where steroids fall short. For infectious disease, researchers now test broadly neutralising antibodies to curb emerging variants. Unmet clinical needs therefore reinforce global demand for these biologics.
Government and public institutions have become essential partners in advancing antibody science. The United States National Institutes of Health and the European Commission’s Horizon programme now allocate sizeable grants. Similar agencies in Asia follow the same path. Their funding supports work on antibody‑drug conjugates and long‑acting formulations. At the same time, regulators are smoothing the path to market. The U.S. Food and Drug Administration and the European Medicines Agency offer accelerated review lanes, and Asian authorities have joined them. These routes shorten clinical timelines, cut costs, and give patients earlier access. As a result, the pipeline remains very robust and competitive.
Broader access and smarter manufacturing are strengthening long‑term growth. The World Health Organization now promotes biosimilar antibodies to improve affordability in low‑ and middle‑income countries. Several emerging economies, including India, Brazil, and Egypt, are expanding local bioreactor capacity and adopting global quality standards. Regional production lowers import dependence, cuts lead times, and stabilises supply during crises. Meanwhile, technology advances such as single‑use systems, continuous processing, and AI‑guided quality control are driving down cost per gram. As these efficiencies take hold, payers can reimburse more indications. Providers can stock reliable inventories, and patients gain faster, sustained access to critical antibody care.
Key Takeaways
- A market analyst noted that the global antibody therapy sector is forecasted to jump from US$ 231.6 Billion in 2023 to US$ 669.1 Billion by 2033.
- According to experts, monoclonal antibodies dominated the antibody therapy segment in 2023, capturing more than 69.1% of the overall market share.
- Industry insiders observed that hospitals remained the top end-users in 2023, representing approximately 47.4% of all antibody therapy applications.
- It was reported that North America led the regional markets in 2023, contributing 38.9% to global revenues, with a valuation of US$ 90.1 million.
Segmentation Analysis
Type Analysis
In 2023, Monoclonal Antibodies dominated the Antibody Therapy Market’s Type Segment with over 69.1% share. This is due to their wide use in treating cancer, autoimmune diseases, and infections. In oncology, they target and neutralize cancer cells with high accuracy. This makes them more effective than many traditional therapies. In autoimmune disorders, they selectively block malfunctioning immune components. This helps manage symptoms more efficiently. Their role in infectious diseases is also growing. These antibodies target harmful pathogens, offering a focused and safer treatment approach.
End User Analysis
Hospitals led the End User Segment in 2023, capturing over 47.4% of the market. They offer the infrastructure and skilled staff needed for advanced antibody therapies. Specialty centers are also important in delivering targeted treatments. They are especially vital in cancer and autoimmune care. Clinics and research facilities support the market through clinical trials and innovation. These organizations help develop new treatment protocols. The market will keep evolving. Hospitals will likely stay dominant, while specialty centers will grow due to rising demand for personalized care.
By Type
- Monoclonal Antibodies
- Oncology, Autoimmune Disease
- Infectious Disease
- Other
- Antibody Drug Conjugates
By End User
- Hospitals
- Specialty Centers
- Others
Regional Analysis
In 2023, North America led the antibody therapy market with over 38.9% share, reaching a market value of US$ 90.1 million. This strong position is supported by the region’s advanced healthcare systems and high investment in research and development. The U.S. plays a central role, driven by its strong biotechnology sector and increasing cases of cancer and autoimmune diseases. These factors boost the demand for targeted treatments. A well-structured regulatory environment also enables faster approvals of new therapies, helping the market grow rapidly across the region.
Canada also contributes significantly to North America’s market leadership. Its supportive government policies promote innovation in healthcare and biopharma research. The country benefits from strong partnerships between universities and biotech companies. These collaborations foster the development of new therapies. Canada’s focus on personalized medicine and innovation adds to the region’s overall strength. Together with the U.S., North America remains a hub for antibody therapy progress, offering strong potential for future growth and technological breakthroughs.
Key Players Analysis
The antibody therapy market is highly competitive, dominated by global leaders like F. Hoffmann-La Roche Ltd. and AbbVie Inc., known for Rituxan and Humira. Amgen Inc. and Bristol-Myers Squibb are growing in oncology and autoimmune therapies. Johnson & Johnson benefits from products like Darzalex and Stelara. Emerging companies focus on niche therapies and personalized medicine. Smaller firms and regional players offer cost-effective solutions. Market strength is reinforced by robust R\&D, global networks, and innovative pipelines, ensuring long-term growth and strong brand positioning for leading industry participants.
Key strategies include mergers, acquisitions, and collaborations to boost product portfolios and expand into new markets. Companies are investing in advanced manufacturing to enhance antibody production. Partnerships with universities and tech firms are driving innovation, especially in oncology and autoimmune care. The rise of biosimilars is reducing costs and countering patent expirations. Firms are also exploring rare diseases, immune-oncology, and bi-specific antibodies. Despite pricing pressures and patent risks, the market outlook remains positive, driven by high demand and continuous innovation.
- F. Hoffmann-La Roche Ltd.
- AbbVie Inc.
- Amgen Inc.
- Bristol-Myers Squibb Company
- Johnson & Johnson Services Inc.
- Seagen
- Merck & Co. Inc.
- Regeneron Pharmaceuticals Inc.
- Novartis AG
- AstraZeneca
- Eli Lilly and Company
- GlaxoSmithKline plc.
- Takeda Pharmaceutical Company Ltd.
Emerging Trends
Faster Approvals and Regulatory Support
Regulatory agencies are speeding up the approval process for antibody-based treatments. This change allows new drugs to reach patients faster than before. Faster approvals are especially important for life-threatening conditions like cancer or autoimmune diseases. Authorities like the U.S. FDA and European Medicines Agency are offering special pathways such as priority review or accelerated approval. These programs help pharmaceutical companies reduce development timelines. As a result, patients benefit from earlier access to innovative treatments. This trend is helping fuel global market growth and encouraging investment in antibody research. Faster regulatory support also boosts trust in the safety and effectiveness of new therapies.
AI Is Changing How Antibodies Are Made
Artificial intelligence (AI) is now helping scientists design new antibody therapies. AI can predict how an antibody will behave in the human body. This means fewer failed experiments and faster results. It also helps researchers choose the best candidates for clinical trials. With AI, companies can find better targets, shorten development time, and lower costs. This trend is changing the way drug discovery works. It brings more accuracy and speed to antibody research. Over the next few years, AI is expected to play a key role in making new therapies better and safer.
Easier and More Convenient Treatments
A major trend in antibody therapy is making treatment easier for patients. Many companies are now creating therapies that can be injected at home instead of in hospitals. This is a big shift from traditional infusions that required clinic visits. Subcutaneous (under-the-skin) injections are now being developed for several antibody drugs. These are simpler, quicker, and less stressful. It helps patients stick to their treatment plans and lowers hospital costs. This change also benefits people with chronic diseases who need long-term care. Convenience and comfort are now a key focus in therapy design.
The Rise of Biosimilars
Biosimilars are low-cost versions of older antibody drugs whose patents have expired. They offer the same medical benefits at a lower price. This is a big deal for healthcare systems and patients. With rising medical costs, biosimilars provide a more affordable option. They also help expand access in lower-income regions. The market for biosimilars is growing fast, especially in Europe and Asia. Companies are investing in developing biosimilars for well-known drugs like Humira and Rituxan. As more biosimilars become available, the overall cost of antibody therapy is expected to drop, improving affordability worldwide.
Use Cases
Cancer Treatment
Antibody therapy is widely used to treat various types of cancer. It works by targeting cancer cells directly, without damaging healthy ones. This makes it different from chemotherapy, which affects both good and bad cells. As a result, patients experience fewer side effects. Antibodies can also help the immune system recognize and attack cancer cells. Treatments are more precise and often more effective. This method is especially helpful in conditions like breast cancer, lymphoma, and leukemia. Newer formats like bispecific antibodies are also showing great promise in clinical trials. Antibody therapy is becoming a key part of modern cancer care.
Autoimmune Disease Management
Antibody therapy is helping people manage autoimmune diseases. Conditions like rheumatoid arthritis, lupus, and multiple sclerosis respond well to these treatments. Antibodies work by calming down the overactive immune system. This reduces inflammation and pain. It also helps protect the body’s healthy tissues from damage. Many patients experience better mobility and quality of life. These therapies are often safer and more targeted than traditional immune-suppressing drugs. Some are taken by injection every few weeks. Others are given through an IV. Antibody therapy continues to improve long-term outcomes in autoimmune conditions.
Alzheimer’s Disease and Brain Health
New antibody therapies aim to slow Alzheimer’s disease. These treatments target harmful proteins in the brain, such as beta-amyloid. These proteins are believed to cause memory loss and brain damage. By removing or blocking them, antibodies may help slow down disease progression. They don’t cure Alzheimer’s, but they may help people stay independent longer. Some patients show better memory and thinking skills for a time. These treatments are mostly used in early-stage patients. While more research is needed, this approach offers hope for brain health in aging populations.
Infectious Disease Prevention
Antibodies are used to fight infections like COVID-19 and RSV. They help people who are at high risk, including seniors and those with weak immune systems. These treatments can prevent severe illness or help the body recover faster. Some antibodies are given as a preventive shot. Others are used after exposure or early symptoms. During the COVID-19 pandemic, they saved lives when vaccines weren’t enough. Antibody therapies are also being explored for flu and other viruses. They provide a critical tool in protecting public health, especially during outbreaks or in vulnerable groups.
Conclusion
Antibody therapy is on a strong upward path and still has room to grow. Demand stems from its precise way of treating cancer, autoimmune illness, and stubborn infections while sparing healthy cells. Funding bodies and regulators now work together to move new molecules from lab to bedside faster than before. Firms invest in smarter factories and digital tools so costs fall and access widens. Biosimilars will further open the door for payers and patients in every region. Because science, policy, and production now align, the therapy stands ready to anchor modern care. It will shape the next wave of targeted medicine.


