Table of Contents
Overview
New York, NY – July 24, 2025 – The Global RNAi Technology Market Size is expected to be worth around US$ 7.7 Billion by 2033, from US$ 2.3 Billion in 2023, growing at a CAGR of 12.8% during the forecast period from 2024 to 2033. North America dominated the RNAi Technology Market, accounting for over 35.82% of the market share and achieving a valuation of US$ 0.8 billion in the year.
The global RNA interference (RNAi) technology market is experiencing accelerated growth, driven by increasing investments in gene-silencing therapies and the expanding landscape of precision medicine. RNAi, a natural biological process that inhibits gene expression, is now being harnessed to develop targeted treatments for a wide range of diseases, including cancer, viral infections, and genetic disorders.
In 2023, RNAi-based drugs such as Patisiran and Givosiran continued to demonstrate positive outcomes in treating hereditary transthyretin amyloidosis and acute hepatic porphyria, respectively. These successes have catalyzed further clinical trials and regulatory approvals. Moreover, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are providing accelerated pathways for RNAi therapies, encouraging new entrants and product development.
Technological advancements in delivery platforms especially lipid nanoparticles (LNPs) have improved the precision and safety of RNAi therapeutics, making systemic delivery more efficient. As a result, biopharmaceutical companies are increasingly partnering with academic institutions to scale RNAi pipelines and diversify clinical indications.
North America remains the leading region due to robust research infrastructure and regulatory support, while Asia-Pacific is emerging rapidly with increasing R\&D initiatives and patient enrollment in RNAi clinical studies. With its potential to silence disease-causing genes at the molecular level, RNAi technology is set to transform the future of medicine.
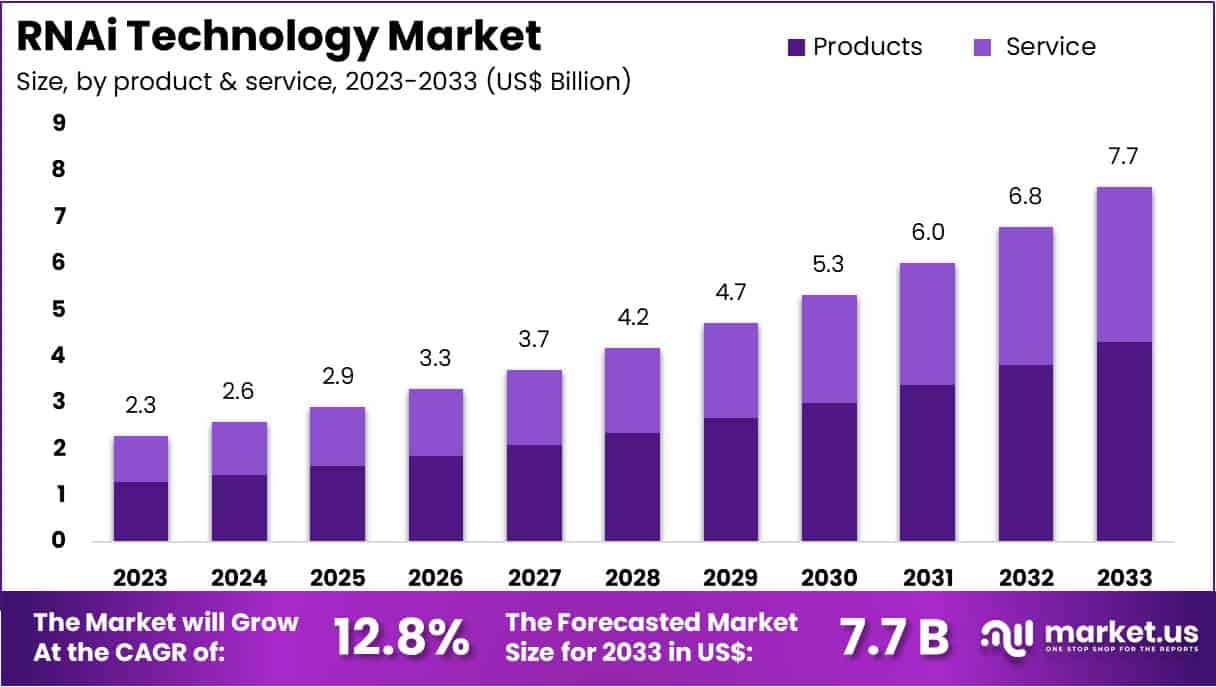
Key Takeaways
- The global RNAi Technology Market is expected to grow from US$ 2.3 billion in 2023 to US$ 7.7 billion by 2033, registering a compound annual growth rate (CAGR) of 12.8% over the forecast period.
- In 2023, the Products segment accounted for the largest share in the Product & Service analysis, representing over 56.41% of the total market.
- Based on application, the Drug Discovery & Development segment dominated the RNAi Technology Market in 2023, contributing 38.46% of the overall market share.
- By end-user, Pharmaceutical and Biotechnology Companies emerged as the primary contributors in 2023, holding a market share of 49.57%.
- North America led the regional landscape of the RNAi Technology Market in 2023, capturing 35.82% of the market and reaching a market value of US$ 0.8 billion.
Segmentation Analysis
- Product & Service Analysis: In 2023, the Products segment dominated the RNAi Technology Market, holding over 56.41% share. This includes miRNA and siRNA products, widely used in gene expression studies, diagnostics, and therapeutics. miRNAs are essential in disease regulation, while siRNAs enable precise gene silencing. The “Others” category includes emerging RNAi tools. Services such as gene synthesis and delivery optimization complement product usage, enhancing R&D efficiency and accelerating RNAi adoption across commercial and academic research sectors.
- Applications Analysis: The Drug Discovery & Development segment led the applications segment in 2023, with over 38.46% market share. RNAi plays a key role in silencing target genes, supporting disease pathway analysis and new drug identification. Therapeutic applications, notably in oncology, ocular, and respiratory disorders, use RNAi to develop targeted treatments. Functional genomics and agricultural applications also contribute to market growth by enhancing genetic understanding and improving crop resilience through gene expression modifications.
- End-user Analysis: Pharmaceutical and biotechnology companies accounted for over 49.57% of the end-user market in 2023, driven by high R&D spending and the adoption of RNAi in therapeutic development. These firms utilize RNAi for large-scale drug discovery and gene therapy initiatives. Academic and research institutes follow, focusing on gene function studies supported by public and private funding. Contract Research and Manufacturing Organizations (CROs and CMOs) also contribute by offering outsourced RNAi-based drug development and production services.
Market Segments
By Product & Service
- Products
- miRNA
- siRNA
- Others
- Service
By Applications
- Drug Discovery & Development
- Therapeutics
- Oncology
- Ocular Disorders
- Respiratory Disorders
- Hepatitis B and C
- Autoimmune Hepatitis
- Neurological Disorders
- Other Therapeutics
- Functional Genomics
- Others
By End-user
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- CROs & CMOs
Regional Analysis
In 2023, North America dominated the RNAi Technology Market, accounting for over 35.82% of the global share and achieving a market value of US$ 0.8 billion. This leadership is attributed to the region’s strong biomedical research ecosystem, substantial biotechnology investments, and favorable regulatory policies that promote innovation in genetic and pharmaceutical research.
The United States remains the primary contributor, supported by advanced healthcare infrastructure and the presence of leading RNAi-focused companies. These factors have facilitated the swift adoption of RNAi technologies in both therapeutic development and clinical applications.
Public and private sector funding across North America continues to drive research in RNAi-based treatments, particularly targeting cancer and chronic diseases. Canada and Mexico, while smaller in market size, are making strategic advancements in healthcare and biotechnology research, contributing to regional growth.
With ongoing technological innovation, increased research activity, and supportive policy frameworks, North America is expected to maintain its leading position in the RNAi Technology Market over the forecast period.
Emerging Trends
- Increasing Approvals of RNAi Therapeutics: The growth of RNAi technology can be attributed to a steady stream of new drug approvals by the U.S. Food and Drug Administration (FDA). To date, more than ten small interfering RNA (siRNA)–based therapies have received FDA approval, marking a transition from concept to clinical reality in rare and common diseases alike.
- Advances in Targeted Delivery Methods: The adoption of Nacetylgalactosamine (GalNAc) conjugation has enabled highly efficient, liver targeted delivery of siRNAs. Early clinical studies demonstrated that single and multiple subcutaneous doses of GalNAc conjugated siRNAs were well tolerated in over 40 healthy volunteers, paving the way for lower dosing frequencies and improved safety profiles.
- Expansion of the Clinical Trial Pipeline: A robust pipeline of RNAi candidates is under evaluation, with hundreds of active studies registered on ClinicalTrials.gov. These encompass Phase I through Phase III trials across diverse indications ranging from genetic disorders to cardiovascular and neurological diseases reflecting broad confidence in RNAi as a therapeutic platform.
- Diversification into Agricultural Applications: Beyond medicine, RNAi is being harnessed in crop protection. Government funded research has demonstrated that RNAi based sprays can effectively silence pest genes, achieving mortality rates above 70% in targeted insect species and offering a biodegradable alternative to chemical pesticides.
Use Cases
- Hereditary Transthyretin Mediated Amyloidosis: Patisiran (Onpattro) was approved in 2018 for adults with hATTR amyloidosis, reducing transthyretin protein levels by up to 87% and demonstrating a favorable safety profile in pivotal trials.
- Primary Hyperoxaluria Type 1: Lumasiran received FDA approval for PH1, with Phase III data showing a mean urinary oxalate reduction of 65% in a cohort of pediatric and adult patients, underscoring RNAi’s potential in rare metabolic disorders.
- Atherosclerotic Cardiovascular Disease: In a Phase II trial of olpasiran (AMG 890) for elevated lipoprotein(a), 480 participants were randomized, demonstrating up to a 50% reduction in Lp(a) levels at 24 weeks, signaling promise for cardiovascular risk reduction.
- Alcohol Use Disorder: DCR AUD is an ongoing 24 week, Phase I/II study evaluating an RNAi agent for alcohol use disorder in adults, representing one of the first investigations of RNAi in behavioral health indications.
- Crop Pest Management: Field trials funded by national agriculture agencies have shown that topical RNAi formulations can achieve over 70% mortality in key insect pests across multiple crop species, offering a targeted, environmentally friendly alternative to traditional insecticides.
Conclusion
The RNAi Technology Market is poised for transformative growth, underpinned by expanding clinical applications, rising therapeutic approvals, and innovations in targeted delivery. With a projected CAGR of 12.8% from 2023 to 2033, the market is witnessing significant adoption across pharmaceuticals, functional genomics, and agriculture.
North America leads due to strong R&D infrastructure and regulatory support, while global interest is broadening. Increasing clinical trials and emerging use cases in rare diseases and crop protection further validate RNAi’s potential. As gene-silencing technologies mature, RNAi is expected to become a cornerstone in next-generation therapies and sustainable biotechnological solutions worldwide.


