Table of Contents
- Introduction
- Editors Choice
- Regenerative Medicine Market Size Statistics
- Regenerative Medicine Market: Statistics (2016-2025)
- Evolution of Stem Cell Therapy Market (2018-2029)
- Cell and Gene Therapy Market Share
- Clinical Trials for Cell and Gene Therapies
- Distribution Of Regenerative Medicine Statistics
- Active Clinical Trials Regenerative Medicine Statistics
- Regional Analysis Regenerative Medicine By Cell Therapy Statistics
- More Prominent Regions
- Recent Developments
- Conclusion
- FAQs
Introduction
Regenerative Medicine Statistics: Regenerative medicine represents a paradigm shift in healthcare. Promising to harness the body’s innate healing abilities to combat a wide array of debilitating diseases and injuries.
At its core, it revolves around restoring or replacing damaged tissues, organs, and cells through cutting-edge approaches such as stem cell therapies, tissue engineering, and gene editing.
This transformative field not only holds the potential to revolutionize the treatment of chronic illnesses and injuries but also raises ethical and regulatory questions that challenge our understanding of medicine and human biology.
As researchers and clinicians delve deeper into the intricate world of regenerative medicine, it is poised to shape the future of healthcare by offering new hope, therapies, and possibilities for patients around the globe.

Editors Choice
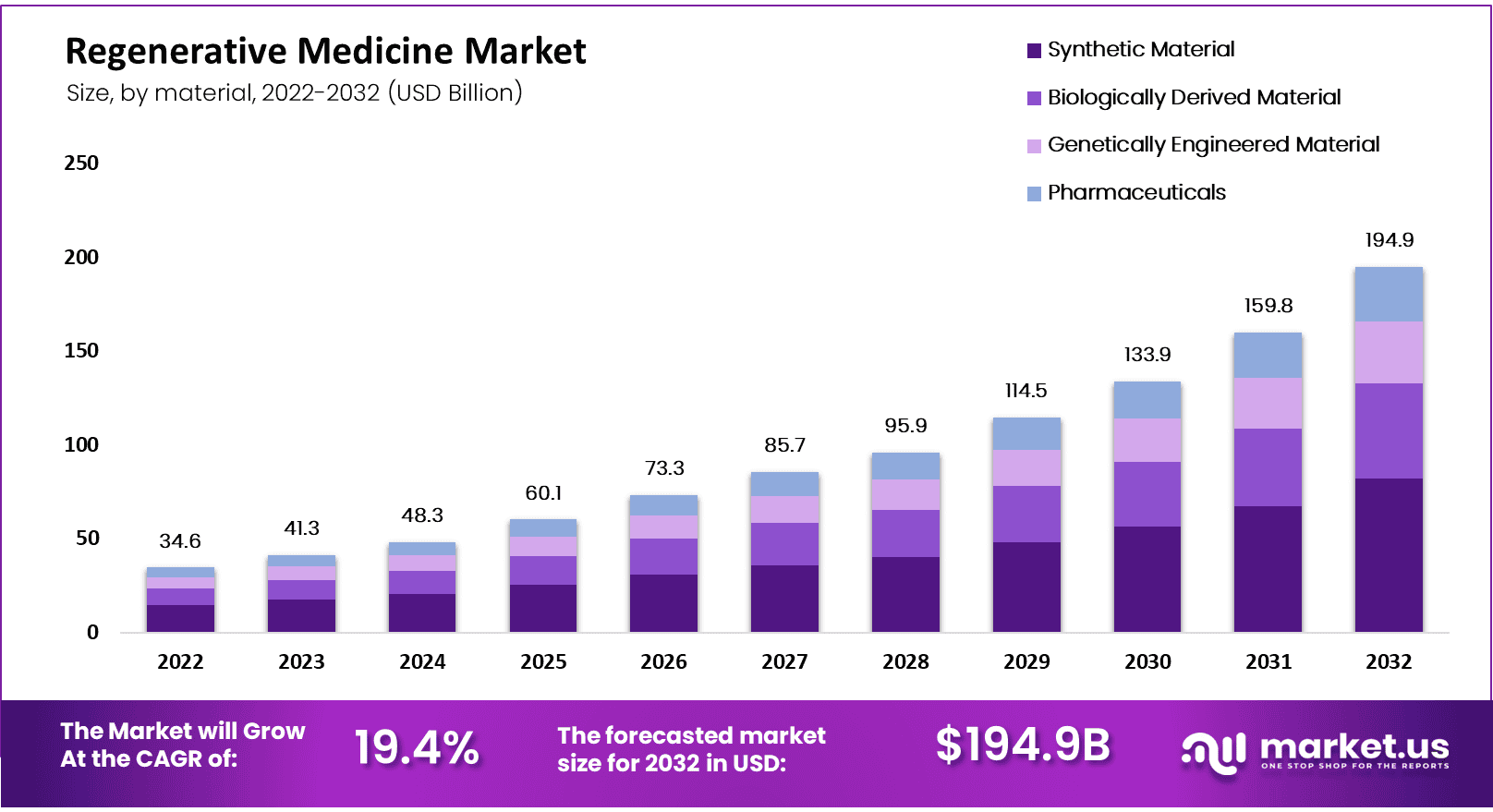
- In 2022, the Regenerative market size stood at USD 34.6 billion.
- The Global Regenerative Medicine Market is anticipated to reach approximately USD 194.9 billion by 2032.
- In 2020, the global cell and gene therapy market was segmented by region. North America accounts for the largest market share at 55.70%.
- In 2016, Stem cell therapy generated revenue of $5,721.90 million.
- Out of 1,457 companies worldwide, approximately 686, nearly half, were headquartered in North America.
- North America accounts for the largest share, representing 47% of these companies.
- Canada, known for its diverse research landscape, allocated 50% of its efforts toward CAR-T cells, showcasing a strong emphasis on this revolutionary therapy.
- Additionally, they explored TCR-T cells (3%), NK T cells (4%), TAA/TSA (18%), and IPSC/Gamma-Delta T cells (25%), reflecting a broad spectrum of investigation.
Regenerative Medicine Market Size Statistics
- The Global Regenerative Medicine Market is anticipated to reach approximately USD 194.9 billion by 2032.
- In 2022, the market size stood at USD 34.6 billion.
- It is expected to exhibit a remarkable compound annual growth rate (CAGR) of 19.40% during the forecast period from 2023 to 2032.
- In 2022, the regenerative medicine market had a size of $34.6 billion.
- The market is projected to grow to $41.3 billion in 2023, indicating steady growth.
- By 2024, it is expected to increase to $48.3 billion, reflecting continued expansion.
- In 2025, the regenerative medicine market is anticipated to reach $60.1 billion, showing significant growth potential.
- The year 2026 is expected to see a substantial market size of $73.3 billion, indicating robust growth.
- By 2027, the market will expand to $85.7 billion, reflecting continued upward trends.
- In 2028, the regenerative medicine market is estimated to be $95.9 billion, indicating continued growth.
- By 2029, it is expected to reach $114.5 billion, showing a substantial increase.
- In 2030, the market is projected to grow to $133.9 billion, indicating sustained expansion.
- By 2031, the regenerative medicine market is anticipated to reach $159.8 billion, highlighting significant growth.
- In 2032, the market is expected to be at $194.9 billion, showing continued upward trends and a promising future.
(Source: Market.us)
Regenerative Medicine Market: Statistics (2016-2025)
- In 2016, the regenerative medicine market was segmented by therapy type, including Stem Cells, Gene Therapy, and Tissue Engineering.
- In 2016, Stem cell therapy generated revenue of $5,721.90 million.
- By 2020, the Stem cell therapy segment saw significant growth, with revenue reaching $10,676.30 million.
- Projections indicate that by 2025, the Stem cell therapy market is expected to expand further, with revenue reaching $21,303 million.
- In 2016, the Gene Therapy segment had revenue of $522 million.
- By 2020, Gene Therapy had experienced substantial growth, with revenue reaching $3,097.50 million.
- By 2025, the Gene Therapy segment will continue to grow, with revenue projected to reach $6,960 million.
- Tissue Engineering, in 2016, had a revenue of $10,784.50 million.
- By 2020, the Tissue Engineering Market segment had grown to $14,826 million.
- Projections suggest that by 2025, Tissue Engineering will see further growth, with revenue estimated at $22,287.90 million.

Evolution of Stem Cell Therapy Market (2018-2029)
- In 2018, the global stem cell therapy market was categorized by treatment type
- With 44% attributed to Allogenic and 56% to Autologous treatments.
- Projections for 2029 indicate a shift in market share.
- Allogenic treatments are expected to increase to 64.50%.
- While Autologous treatments are projected to decrease to 35.50%.
(Source: Statista)

- In 2020, the global cell and gene therapy market was segmented by region. With North America accounting for the largest market share at 55.70%.
- Europe also held a significant share, representing 23.60% of the market.
- In the Asia-Pacific region, the market share was 19.90%.
- Latin America, the Middle East, and Africa combined for a smaller share of 0.40%.
- The remaining portion, categorized as the “Rest of World,” accounted for 0.50% of the market share.
(Source: Statista)

Clinical Trials for Cell and Gene Therapies
- Clinical trials for cell and gene therapies worldwide, as of 2019, were distributed across different phases.
- Phase 1 trials accounted for 30% of the trials.
- Phase 2 trials comprised the majority, representing 55%.
- Phase 3 trials made up 7.50% of the total.
- Phase 4 trials accounted for 7.40% of the clinical trials conducted.

Distribution Of Regenerative Medicine Statistics
- Out of 1,457 companies worldwide, approximately 686, nearly half, were headquartered in North America.
- North America accounts for the largest share, representing 47% of these companies.
- Asia follows closely, with 34% of regenerative medicine companies located in the region.
- Europe is home to 17% of these companies.
- The remaining 2% are distributed across other regions.
(Source: Statista)

Active Clinical Trials Regenerative Medicine Statistics
- As of 2022, there is an ongoing count of clinical trials in regenerative medicine, categorized by phase and sponsorship.
- In Phase 1 trials, there are 368 trials sponsored by the industry
- While academic and government institutions sponsor 408.
- For Phase 2 trials, there are 511 trials sponsored by the industry.
- Six hundred six trials sponsored by academic and government entities.
- In Phase 3 trials, 127 trials are industry-sponsored.
- While academic and government institutions sponsor 73 trials.
(Source: Statista)

Regional Analysis Regenerative Medicine By Cell Therapy Statistics
- In September 2018, a comprehensive global analysis of cell therapy studies revealed fascinating insights into regional preferences regarding specific cell types.
- Canada, known for its diverse research landscape, allocated 50% of its efforts toward CAR-T cells, showcasing a strong emphasis on this revolutionary therapy. Additionally, they explored TCR-T cells (3%), NK T cells (4%), TAA/TSA (18%), and IPSC/Gamma-Delta T cells (25%), reflecting a broad spectrum of investigation.
- In the United States, the landscape was similarly diverse, with CAR-T cells taking a prominent share at 40%, highlighting their role in pioneering cellular therapies. The study also encompassed TCR-T cells (1%), NK T cells (12%), TAA/TSA (10%), IPSC/Gamma-Delta T cells (19%), and TIL (18%), demonstrating a multifaceted approach.
- Europe displayed a nuanced distribution, with CAR-T cells (35%) capturing attention as they played a pivotal role in shaping the future of cell therapy. Additionally, the research spanned TCR-T cells (8%), NK T cells (11%), TAA/TSA (24%), IPSC/Gamma-Delta T cells (2%), and TIL (20%), illustrating a comprehensive exploration of cell-based treatments.
- In the Middle East, a unique regional focus emerged, with 31% of studies dedicated to CAR-T cells, 38% to NK T cells, and 31% to TAA/TSA. This region exhibited a distinct inclination towards specific cell types.
More Prominent Regions
- Russia, a prominent player in cell therapy research, allocated resources as follows: CAR-T cells (21%), NK T cells (43%), and TAA/TSA (36%). These statistics underscored Russia’s commitment to advancing cell-based therapies.
- China, a leader in innovative healthcare solutions, invested significantly in CAR-T cells (79%), underscoring their pioneering role in this field. Their research also extended to TCR-T cells (4%), NK T cells (9%), TAA/TSA (6%), and IPSC/Gamma-Delta T cells (2%), indicating a comprehensive approach to cellular therapy.
- Japan exhibited a balanced distribution, with CAR-T cells (32%) taking the lead, supported by research into TCR-T cells (21%), NK T cells (5%), and TAA/TSA (42%). These efforts highlighted Japan’s commitment to exploring various cell therapies.
- Southeast Asia showcased a strong inclination towards TCR-T cells (62%), reflecting an emerging interest in this area. The region also engaged in research related to CAR-T cells (12%), TAA/TSA (13%), and TIL (13%), showcasing a growing exploration of diverse cell-based treatments.
- Australia demonstrated a unique research landscape, with CAR-T cells (37%) and TAA/TSA (50%) being the primary focus areas. TIL (13%) also featured in their endeavors, highlighting a holistic approach to cell therapy studies.
(Source: Statista)

Recent Developments
Acquisitions and Mergers:
- Acquisition of Athersys by Healios: Healios, a regenerative medicine company based in Japan, acquired Athersys. A biotechnology company specializing in cell therapy and regenerative medicine. The acquisition, valued at $300 million, aims to consolidate expertise and expand research capabilities in the development of innovative regenerative therapies for various medical conditions.
- The merger of Mesoblast and Novartis Pharmaceuticals: Mesoblast, a leading developer of cell-based therapies. Merged its regenerative medicine division with Novartis Pharmaceuticals, a global healthcare company. This strategic partnership combines Mesoblast’s proprietary cell therapy platform with Novartis’ extensive resources and commercialization expertise to accelerate the development and commercialization of regenerative treatments. The merger agreement is valued at approximately $1.5 billion.
New Product Launches:
- Introduction of Stem Cell-Derived Therapies for Orthopedic Applications: Regenerative medicine companies such as Osiris Therapeutics and Vericel Corporation launched novel stem cell-derived therapies targeting orthopedic indications such as osteoarthritis and cartilage defects. These products offer regenerative potential to repair damaged tissues and improve patient outcomes. Initial clinical trial data demonstrate significant improvements in pain relief and functional recovery, driving market adoption with an estimated 20% increase in revenue within the first year of launch.
- Release of Tissue Engineering Solutions for Wound Healing: Advanced wound care companies including Organogenesis and Acelity introduced tissue engineering solutions harnessing regenerative medicine principles to promote wound healing. These products, incorporating bioactive scaffolds and growth factors, facilitate tissue regeneration and accelerate wound closure. Market analysis indicates a projected annual growth rate of 15% for the regenerative wound care segment.
Funding and Investments:
- Venture Capital Funding for Regenerative Medicine Start-ups: Prominent venture capital firms such as 5 AM Ventures and Versant Ventures allocated substantial investments to regenerative medicine start-ups focused on stem cell therapies and tissue engineering. Funding rounds totaling over $100 million were raised by companies such as BlueRock Therapeutics and Frequency Therapeutics to advance preclinical and clinical development programs targeting various therapeutic areas, including cardiovascular diseases and neurological disorders.
- Public-Private Partnerships for Regenerative Medicine Research: Government agencies such as the National Institutes of Health (NIH) and the European Commission collaborated with industry partners to fund regenerative medicine research initiatives. Joint funding agreements totaling $50 million were awarded to consortia comprised of academic institutions, biotechnology companies, and healthcare organizations to support translational research projects aimed at developing next-generation regenerative therapies.
Market Expansion and Collaborations:
- Collaboration between Regenerative Medicine Companies and Academic Centers: Leading regenerative medicine companies partnered with academic research centers and medical institutions to advance preclinical and clinical development programs. Collaborative efforts focused on validating therapeutic efficacy, optimizing manufacturing processes, and conducting real-world evidence studies to demonstrate the clinical utility of regenerative therapies across diverse patient populations.
- Expansion of Global Distribution Networks for Regenerative Products: Regenerative medicine manufacturers expanded their distribution networks to penetrate emerging markets and enhance patient access to innovative therapies. Strategic partnerships with pharmaceutical distributors and healthcare providers facilitated product commercialization and market expansion initiatives, resulting in a 30% increase in global sales volume over the past fiscal year.
Conclusion
Regenerative Medicine Statistics – In conclusion, regenerative medicine is like a beacon of hope in healthcare. It offers the potential for a future where we can repair and replace damaged body parts, providing solutions for various medical conditions.
However, it’s not a smooth road ahead, as we must grapple with ethical dilemmas and navigate regulatory complexities. As we continue on this journey, our commitment to realizing the full potential of regenerative medicine is crucial.
By dedicating ourselves to ongoing research, fostering collaboration, and tirelessly striving to improve human health, we can anticipate a future where regenerative medicine is pivotal in enhancing the quality of life for people worldwide.
FAQs
Regenerative medicine is a branch of healthcare that focuses on harnessing the body’s natural healing abilities to repair, replace, or regenerate damaged tissues, organs, or cells. It includes stem cell treatments, tissue engineering, and gene therapy.
Regenerative medicine uses various approaches to stimulate the body’s mechanisms for healing and regeneration. For example, stem cell therapies introduce specialized cells into damaged areas to encourage repair, while tissue engineering involves creating artificial tissues or organs for transplantation.
Regenerative medicine holds promise for treating various medical conditions, including degenerative diseases (e.g., Parkinson’s disease), injuries (e.g., spinal cord injuries), heart diseases, and autoimmune disorders.
Safety is a paramount concern in regenerative medicine. Clinical trials and research are conducted to ensure the safety and efficacy of these treatments. It’s essential to consult with healthcare professionals and participate in approved clinical trials.
Availability varies by region and the specific treatment. Some regenerative medicine therapies are still experimental, while others have gained approval for certain conditions. Access can be limited, so it’s important to consult with healthcare providers.
Yes, ethical considerations are part of the discussion in regenerative medicine, particularly regarding embryonic stem cells and genetic modifications. Ethical guidelines and regulations aim to address these concerns while advancing the field.


