Table of Contents
Introduction
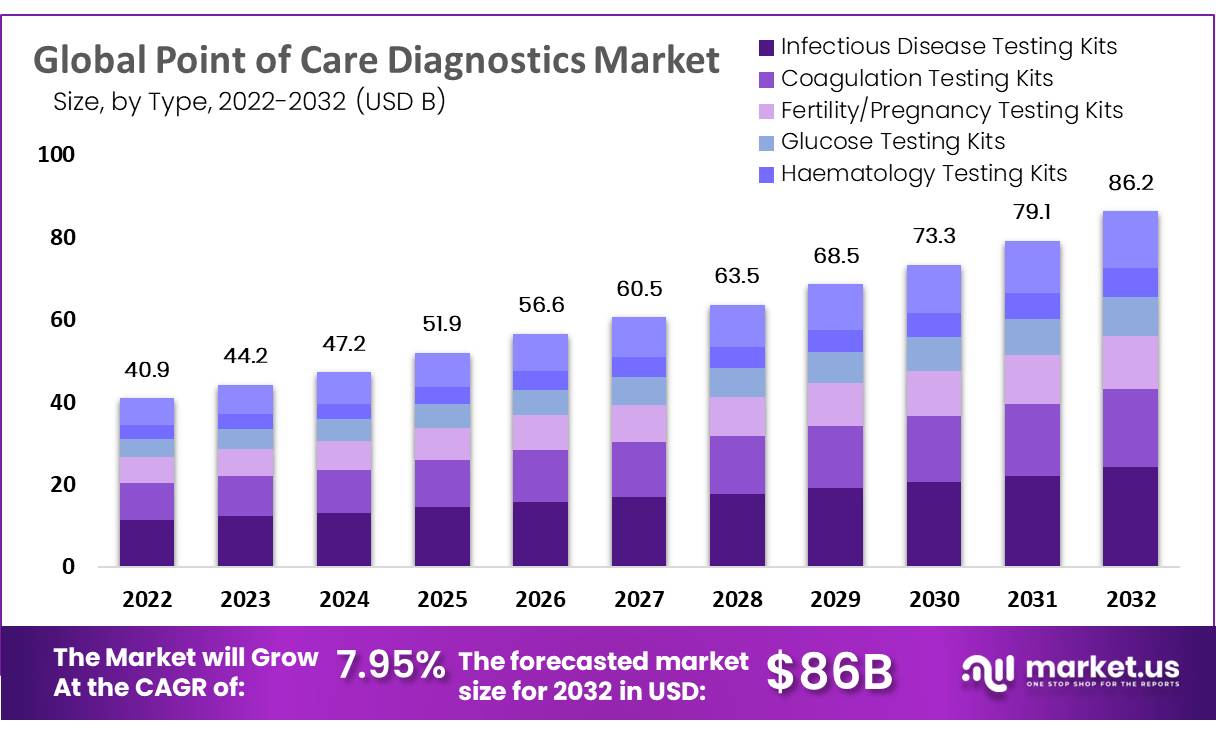
The global Point of Care Diagnostics market is projected to expand from USD 40.9 billion in 2022 to approximately USD 86.2 billion by 2032, achieving a compound annual growth rate (CAGR) of 7.95%. This growth is primarily fueled by the integration of digital health technologies and the increased use of real-world data (RWD) and real-world evidence (RWE).
The COVID-19 pandemic has notably accelerated the demand for rapid diagnostic solutions, further driving development outside traditional lab environments. For instance, initiatives such as the FDA’s Digital and Connected Diagnostics programs play a crucial role in establishing standards for data capture and analysis in non-clinical settings.
Further contributing to the market expansion are enhancements in healthcare programs like Medicaid, which broaden coverage and improve care accessibility. These programs are vital in increasing the demand for PoC diagnostic tools by incorporating more comprehensive diagnostic and monitoring solutions into community and home settings. Such initiatives aim to mitigate health disparities and elevate health outcomes across varied population segments. Additionally, state-specific health surveys, like the Healthy Illinois Survey, offer crucial insights into public health needs and service gaps, guiding investments in health technologies to meet localized health requirements effectively.
Recent developments in the PoC Diagnostics market highlight significant advancements by leading companies such as Roche and Abbott, underscoring their efforts to broaden capabilities and address urgent healthcare needs. For example, Roche’s acquisition of LumiraDx’s Point of Care technology in July 2024, valued at up to $350 million, integrates a versatile multi-assay platform into Roche’s portfolio, enhancing decentralized patient care and access to critical health data.
Moreover, in March 2020, Abbott launched a molecular point-of-care test for COVID-19 on its ID NOW platform, delivering results in under five minutes. This innovation represents a substantial leap in rapid testing, crucial for various healthcare settings, including urgent care clinics and hospital emergency departments. It underscores the company’s commitment to improving the speed and reliability of critical diagnostics during health crises.
Key Takeaways
- Market Growth Overview: By 2032, the global point of care diagnostics market is projected to reach USD 86.2 million, expanding at a CAGR of 7.95% from USD 40.9 billion in 2022.
- Type Analysis: Infectious disease testing kits dominate the market due to their simplicity and suitability for home use.
- End-User Insights: In 2021, clinics were the primary end-users, contributing approximately 35% to the market, with pharmacies and retail clinics as key revenue sources.
- Market Constraints: Growth is hampered by stringent FDA regulations and the prevalence of pre-analytical errors, which restrict new product introductions.
- Opportunities in Emerging Markets: There is significant growth potential in emerging economies, spurred by increasing healthcare investments and infrastructure enhancements.
- Technological Trends: The market is witnessing a shift towards integrating AI and nanotechnology, aiming for greater efficiency and decentralized healthcare solutions.
- Regional Market Dynamics: North America holds the largest market share, followed by Europe. Asia-Pacific is emerging as the fastest-growing region, fueled by an aging population and rising chronic disease rates.
Point of Care Diagnostics Statistics
- Focus on Point-of-Care Diagnostics:
- Point-of-care diagnostics was a target of 164 telemedicine studies in India. It represented 7.5% of the telemedicine interventions.
- Commonly Targeted Diseases:
- Cardiovascular diseases (CVD) accounted for 4.3% of point-of-care diagnostic interventions.
- Cerebrovascular diseases (CeVD) accounted for 1.2% of interventions.
- Diabetes Mellitus (DM) was addressed in 12.2% of interventions.
- Other Health Conditions:
- Hypertension (HTN) was the focus in 7.9% of point-of-care diagnostic studies.
- Infectious diseases (ID) were targeted in 4.9% of interventions.
- Maternal and Child Health (MCH) made up 28.7% of these interventions.
- Additional Conditions Studied:
- Psychiatry conditions represented 10.4% of point-of-care diagnostic studies.
- Oncology (Onc) accounted for 11% of the interventions.
- Ophthalmology (Ophth) represented 11% of the studies.
- Intervention Objectives:
- The primary aim of point-of-care diagnostics was improving health indicators and quality of intervention in various diseases.
Emerging Trends
- Decentralized Testing: Decentralized diagnostics are gaining momentum, enabling medical tests to be conducted outside traditional lab settings. This trend is facilitating access to medical diagnostics in various environments such as homes, workplaces, and even remote regions. By allowing tests to be performed closer to where patients live or work, it reduces the need for travel and wait times, thereby improving patient convenience and care accessibility.
- Integration with Telehealth: There is a growing integration of POC diagnostics with telehealth technologies. This synergy allows for the immediate transfer of medical data to health professionals and enables remote patient monitoring. Such integration not only streamlines the diagnostic process but also enhances patient management by enabling real-time consultations and adjustments in treatment plans based on the latest diagnostic data.
- Advances in Non-invasive Testing: The development of non-invasive diagnostic methods, such as breath tests, is on the rise. These technologies offer a way to detect and monitor diseases without the discomfort of invasive procedures. Non-invasive testing improves patient compliance and comfort, making it easier for patients to undergo regular monitoring without the stress associated with traditional methods.
- Wearable Health Technologies: Wearable devices are increasingly prevalent in the POC diagnostics landscape. These devices facilitate continuous monitoring of various health metrics, providing a constant data flow to healthcare providers. Continuous data monitoring helps in proactive disease management and can lead to more timely interventions, thereby enhancing overall patient outcomes.
- Expansion of Test Menus: The range of tests available through POC diagnostics is expanding significantly. Modern POC devices are not limited to basic tests like blood glucose but now include assessments for markers such as HbA1c, BNP, D-Dimer, and CRP. This expansion enhances the capabilities of POC devices, making them more versatile and valuable in clinical settings.
- Enhanced Connectivity: Modern POC devices offer lab-quality results that can be integrated directly into electronic health records (EHRs). This capability improves the efficiency of data management and facilitates better coordination among healthcare teams. Enhanced connectivity ensures that patient data is easily accessible, up-to-date, and securely stored, which is crucial for effective patient care and management.
Use Cases
- Management of Infectious Diseases: POC diagnostics are instrumental in combatting infectious diseases, particularly in regions with limited medical resources. For instance, quick HIV testing via POC platforms aids global health efforts by ensuring early diagnosis and timely treatment. This application is not only crucial for individual patient health but also helps in controlling the spread of infections, thereby improving overall health outcomes in vulnerable populations.
- Chronic Disease Monitoring: For individuals living with chronic conditions, such as diabetes, POC diagnostics offer a convenient way to regularly monitor vital health parameters like blood glucose levels. This continuous monitoring is essential for managing the disease effectively, allowing for immediate adjustments in treatment plans as needed. It empowers patients to take an active role in managing their health, with real-time data that guides better lifestyle and medication decisions.
- Emergency Medicine Applications: In emergency medical scenarios, the speed at which medical care is provided can be life-saving. POC diagnostics are crucial in these settings as they provide rapid results for critical tests, such as cardiac markers or blood gases. These results are integral to quick decision-making, enabling healthcare professionals to administer the appropriate treatments without delay, thereby improving patient survival rates and recovery times.
- Preventive Health Screening: POC tests play a significant role in preventive health by detecting diseases early in their developmental stages. Early detection through POC screening can lead to significantly better health outcomes by enabling the initiation of treatment much sooner. This aspect of POC diagnostics is particularly important in reducing the long-term impact of potentially severe conditions, highlighting their preventive and proactive benefits in healthcare.
Conclusion
The Point of Care Diagnostics market is expected to experience significant growth over the coming years, driven by advances in digital health technologies and increased use of real-world data. The demand for rapid diagnostic solutions has been accelerated by the COVID-19 pandemic, pushing innovation beyond traditional lab settings. Expanding healthcare programs and the integration of point-of-care tools into home and community environments are further propelling the market. Emerging trends, such as decentralized testing, telehealth integration, non-invasive testing, and wearable health technologies, are transforming the diagnostics landscape. These developments enhance accessibility and efficiency, providing timely and effective care across various settings, ultimately contributing to improved health outcomes and reduced disparities.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



