Table of Contents
Overview
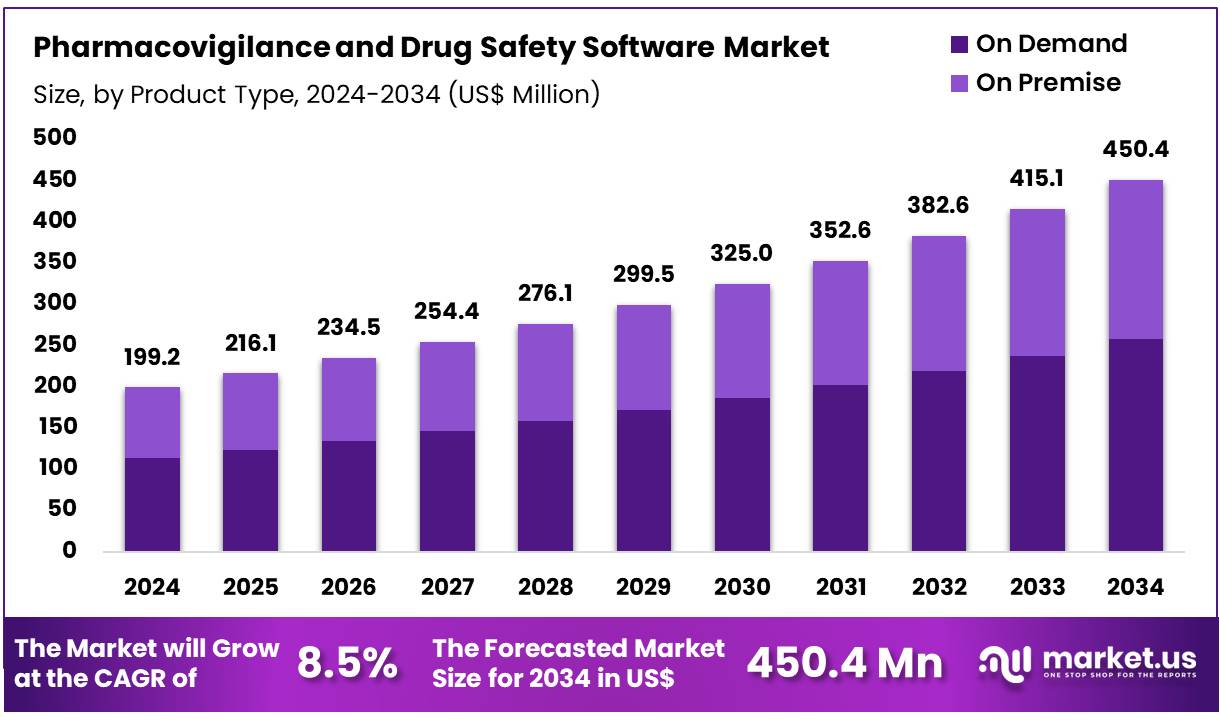
New York, NY – Dec 04, 2025 – Global Pharmacovigilance and Drug Safety Software Market size is expected to be worth around US$ 450.4 Million by 2034 from US$ 199.2 Million in 2024, growing at a CAGR of 8.5% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 36.5% share with a revenue of US$ 72.7 Million.
The global pharmacovigilance and drug safety software market has been experiencing steady expansion as regulatory requirements become more stringent and the volume of adverse event data continues to rise worldwide. Growth in the market has been driven by the increasing need for efficient drug safety monitoring systems, the adoption of advanced digital platforms, and the integration of analytics to support real-time signal detection. The rising complexity of clinical trials and post-marketing surveillance activities has further increased the demand for software solutions capable of managing large datasets with accuracy and compliance.
Pharmaceutical companies, contract research organizations, and regulatory authorities have been implementing automated systems to streamline case processing, ensure data standardization, and enhance reporting efficiency. The adoption of cloud-based architectures has been expanding, as these platforms offer scalability, cost efficiency, and improved accessibility for global operations. The market has also benefited from advancements in artificial intelligence, which have supported automated coding, faster risk assessment, and predictive analytics.
North America has been maintaining a dominant market position due to the presence of established biopharmaceutical industries and strong regulatory frameworks. Meanwhile, Asia-Pacific has been registering notable growth driven by increasing clinical research activity and government initiatives focused on drug safety. The market is expected to continue advancing as organizations prioritize compliance, transparency, and proactive safety management across the drug development lifecycle.
Key Takeaways
- In 2024, the pharmacovigilance and drug safety software market generated revenue of US$ 199.2 million, supported by a CAGR of 8.5%, and the market is projected to reach US$ 450.4 million by 2034.
- The product type segment comprises on-premise and on-demand solutions, with on-demand emerging as the leading category in 2024, accounting for 57.3% of total market share.
- By application, the market is classified into case data collection & management, adverse event reporting & analysis, and signal detection & other safety risk assessment. The signal detection & other safety risk assessment segment held a significant share of 39.6% in 2024.
- In terms of end users, the market includes healthcare companies (pharmaceutical and biotechnology firms), CROs/BPOs or outsourced PV service providers, and other users. The pharmaceuticals and biotechnology companies segment dominated the market with a revenue share of 55.4%.
- Regionally, North America led the global market landscape, securing a 36.5% share in 2024.
Regional Analysis
North America is Leading the Pharmacovigilance and Drug Safety Software Market
North America maintained the highest revenue share of 36.5%, supported by a stringent regulatory framework enforced by the U.S. Food and Drug Administration (FDA) and the rising volume of adverse event submissions. The FDA’s Adverse Event Reporting System (FAERS) has been receiving a substantial number of reports each year, reinforcing the need for advanced software capable of managing, processing, and analyzing expanding datasets. Pharmaceutical companies in the region are required to comply with rigorous pharmacovigilance obligations, including timely adverse event reporting and continuous monitoring of drug safety signals.
The FDA’s strong emphasis on post-marketing surveillance, reflected through active FAERS oversight, has driven the adoption of sophisticated solutions for signal detection, risk assessment, and regulatory compliance. Additionally, the increasing complexity of clinical trials and heightened attention to patient safety have further supported the demand for technologically advanced platforms. Regulatory requirements outlined in 21 CFR Part 314, which mandate structured post-marketing studies and adverse event reporting, continue to highlight the necessity of robust drug safety software across North America.
Asia Pacific Expected to Witness the Highest CAGR During the Forecast Period
The Asia Pacific region is projected to record the fastest CAGR, driven by expanding pharmaceutical manufacturing capabilities and a growing commitment to aligning with global regulatory benchmarks. As regional manufacturers pursue greater participation in international markets, adherence to stringent pharmacovigilance standards has become essential, accelerating the adoption of specialized drug safety software. Strengthening national regulations across several Asia Pacific countries has also contributed to increased emphasis on systematic drug safety monitoring.
Rising reports of adverse drug reactions (ADRs) within the region further necessitate efficient systems for data collection, management, and analysis. Continued investment in healthcare IT infrastructure is expected to support broader deployment of these platforms, enabling improved regulatory compliance and enhanced drug safety oversight. This regulatory harmonization and growing focus on patient safety are predicted to drive sustained demand for pharmacovigilance and drug safety software throughout the Asia Pacific market.
Use Cases
Centralized Collection and Management of Adverse Drug Reaction (ADR) Reports
This use case explains how pharmacovigilance software is used to collect and manage ADR reports from hospitals, physicians, pharmacists, patients, and pharmaceutical companies. The data are standardized through formats such as ICH E2B(R3), allowing direct submission to systems like the FDA’s FAERS and the EMA’s EudraVigilance. The significance of this function is supported by the scale of reporting; the FAERS database contained more than 15 million adverse event reports, with nearly 2 million reports submitted in a single year, while EudraVigilance processed around 1.8 million suspected ADR cases in 2024. Because the volume of safety data runs into millions, the software is used to centralize intake, ensure data quality, and automate de-duplication, making manual systems impractical for global operations.
Early Detection of Drug Safety Signals
In this use case, pharmacovigilance software continuously analyses very large datasets to detect early safety signals by applying statistical methods such as disproportionality analysis. This capability is essential because WHO estimates that one in ten patients is harmed while receiving healthcare, with more than 50% of this harm considered preventable, and medication issues account for a large portion of these events. Hospital studies indicate that 4–10% of inpatients may experience an adverse drug event. Therefore, the software is used to scan incoming data in near real time, helping safety teams identify unusual trends earlier and enabling faster regulatory action or clinical risk mitigation.
Automated Regulatory Reporting and Global Compliance
This use case highlights how software automates the creation, validation, and submission of electronic safety reports to national and international systems such as FAERS in the United States and EudraVigilance in the European Union. It also tracks mandatory reporting timelines and generates periodic safety reports required by global health authorities. The importance of this automation is emphasized by WHO’s global medication safety initiative, which aims to reduce severe, avoidable medication-related harm by 50%. Pharmaceutical companies often need to submit thousands of safety reports annually, and pharmacovigilance software ensures accurate, timely, and compliant reporting across multiple jurisdictions.
Reduction of Medication Errors and Preventable Harm in Healthcare Settings
This use case focuses on how drug safety software integrates with hospital EHRs to capture suspected ADRs and medication errors directly at the point of care. It provides dashboards for identifying high-risk drugs, departments with elevated reporting rates, and trends in preventable harm. WHO estimates the global financial cost of medication errors to be about USD 42 billion annually, representing nearly 1% of global health spending. Some national studies show that up to 12.8% of hospital admissions are linked to ADRs. Because of these high clinical and economic consequences, hospitals use pharmacovigilance systems to analyze medication incidents more systematically, allowing targeted interventions to reduce preventable harm.
Continuous Benefit–Risk Assessment Across the Product Lifecycle
This use case describes how pharmacovigilance software supports ongoing monitoring of the benefit–risk profile of medicines by linking ADR data with real-world usage data. It is especially critical for newly launched medicines or for treatments used in sensitive populations such as children, older adults, and pregnant women. WHO reports emphasize that preventable medication-related harm remains widespread across all healthcare systems, making continuous surveillance necessary. With systems like FAERS and EudraVigilance providing millions of ADR data points each year, the software is used to evaluate safety trends, update risk management plans, and support regulatory decisions regarding labeling changes or additional precautions.
Frequently Asked Questions on Pharmacovigilance and Drug Safety Software
- How does pharmacovigilance software improve drug safety monitoring?
The software improves drug safety monitoring by automating case processing, enabling real-time signal detection, and supporting standardized reporting. These capabilities ensure faster identification of safety risks, improved decision-making, and consistent compliance with global pharmacovigilance regulations and industry guidelines. - Which organizations typically use pharmacovigilance software?
Pharmaceutical companies, biotechnology firms, CROs, regulatory bodies, and healthcare institutions commonly use pharmacovigilance software. These organizations rely on advanced platforms to manage adverse event data efficiently and maintain compliance with mandatory drug safety reporting obligations. - What features are commonly included in pharmacovigilance software?
Key features include case intake and management, automated coding, signal detection tools, regulatory reporting modules, analytics dashboards, and cloud-based accessibility. These functionalities streamline workflows, reduce manual errors, and enable comprehensive drug safety assessments across global operations. - Why is cloud-based pharmacovigilance software gaining popularity?
Cloud-based solutions are gaining traction due to scalability, lower infrastructure costs, and enhanced accessibility. They allow organizations to manage growing safety data volumes efficiently, support remote collaboration, and ensure consistent regulatory compliance across geographically dispersed teams. - Which product type is leading the market?
On-demand software holds the largest market share due to its cost efficiency, flexibility, and ease of deployment. Organizations increasingly prefer cloud-based models to handle expanding datasets and meet evolving regulatory reporting requirements without significant infrastructure investment. - Which region currently dominates the global market?
North America dominates the market owing to strong regulatory frameworks, high adverse event reporting volumes, and significant adoption of advanced drug safety technologies. The region’s established pharmaceutical sector further strengthens demand for comprehensive pharmacovigilance software solutions. - Which segment holds the largest share by application?
The signal detection and safety risk assessment segment holds the largest application share. Its importance is driven by the need for continuous monitoring, early identification of safety issues, and compliance with global regulatory expectations for proactive risk management.
Conclusion
The global pharmacovigilance and drug safety software market has been advancing as regulatory scrutiny intensifies and adverse event data volumes continue to rise. Growth has been supported by increasing adoption of automated, cloud-based, and analytics-driven platforms that enhance reporting accuracy, signal detection, and lifecycle safety evaluation.
North America remains the leading region, while Asia Pacific is projected to expand rapidly due to regulatory harmonization and rising clinical activity. On-demand solutions and signal detection applications hold dominant shares. Overall, market expansion is expected to continue as organizations prioritize compliance, transparency, and proactive safety management across global drug development processes.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



