Table of Contents
Overview
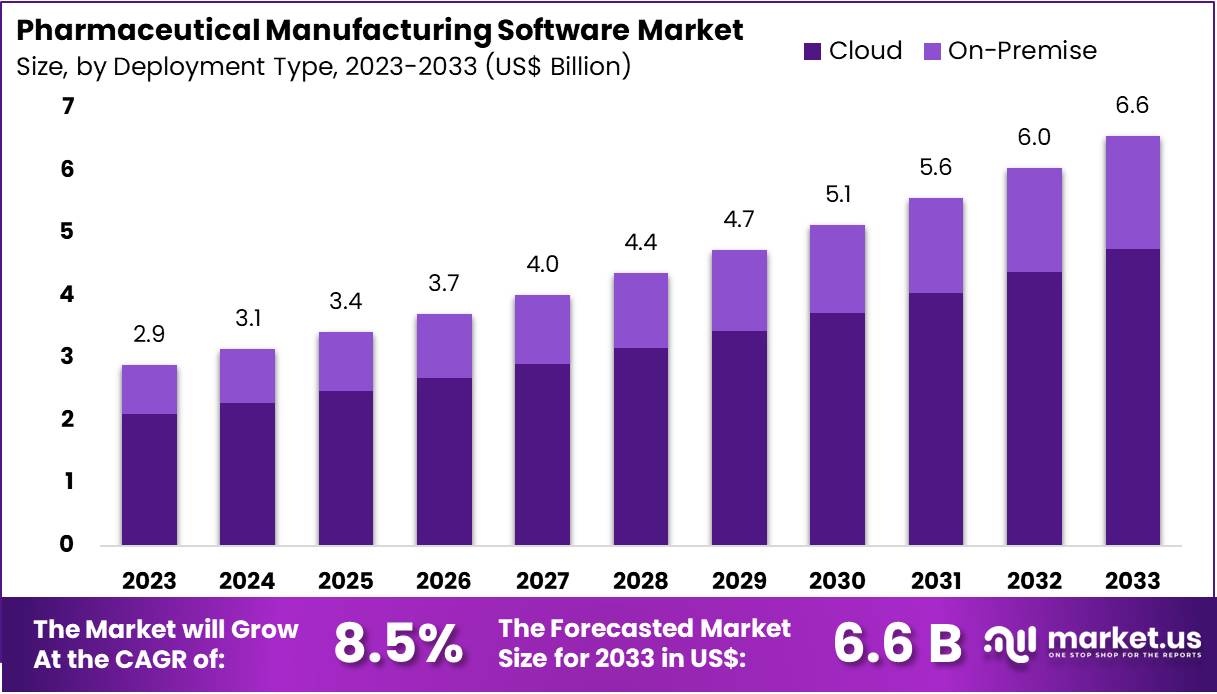
New York, NY – July 21, 2025 : The global pharmaceutical manufacturing software market is expected to reach approximately US$ 6.6 billion by 2033. This growth comes from a market size of US$ 2.9 billion in 2023, showing a strong CAGR of 8.5% during the forecast period. North America leads the market with a 40.7% share, valued at around US$ 1.2 billion in 2023. The region’s dominance is driven by advanced healthcare infrastructure and early adoption of digital technologies. Rising demand for automation and compliance solutions is fueling the global market’s upward trend.
Efficiency, compliance, and product quality are major factors driving market growth. Pharmaceutical companies are under pressure to meet strict regulations while improving operational performance. Software solutions help streamline manufacturing by managing complex processes with precision. These tools support compliance with regulatory bodies like the FDA and EMA. They also enhance data accuracy and reduce risk. As regulatory frameworks grow more stringent, companies increasingly rely on advanced software for consistent quality and smoother audits.
Pharmaceutical manufacturing software is widely used for tasks such as inventory management, batch tracking, and production scheduling. These solutions improve traceability and reduce human error. The growing complexity of drug manufacturing has made manual processes less reliable. Companies now need real-time visibility into operations. This need has driven software adoption across all manufacturing stages. By enhancing process control, these tools help manufacturers maintain high standards, cut costs, and meet deadlines effectively. This shift is reshaping how pharmaceutical firms manage their production systems.
Artificial intelligence (AI) and machine learning (ML) are transforming pharmaceutical manufacturing software. In March 2022, Aizon partnered with Aggity to accelerate digitalization in pharma operations. This reflects a trend toward smart manufacturing solutions. AI-driven tools enable real-time monitoring and predictive maintenance. They also improve quality assurance through data-driven insights. These technologies help identify risks early and boost overall efficiency. With more companies adopting AI, the software market is expected to see continued innovation and growth in the coming years.
Cloud-based pharmaceutical manufacturing software is gaining popularity. These solutions offer easy integration with enterprise systems, secure data storage, and scalability. They support remote access, enabling better collaboration and decision-making. Cloud platforms also reduce IT costs and simplify updates. As the industry moves toward digital transformation, cloud-based tools are becoming essential. They support flexible manufacturing models and faster product rollouts. The growing adoption of these technologies will likely play a key role in expanding the pharmaceutical manufacturing software market over the next decade.
Key Takeaways
- In 2023, the pharmaceutical manufacturing software market recorded revenue of US$ 2.9 billion and is projected to reach US$ 6.6 billion by 2033.
- The market is growing steadily at a compound annual growth rate (CAGR) of 8.5% over the forecast period from 2024 to 2033.
- Cloud-based deployment dominated in 2023, capturing 72.5% of the market share due to its scalability, remote access, and reduced infrastructure costs.
- On-premise deployment lags behind, as more pharmaceutical companies shift toward flexible and cost-effective cloud software solutions.
- Large enterprises made up 67.9% of the market, leveraging advanced software to streamline operations, ensure compliance, and maintain production efficiency.
- Small and medium-sized enterprises (SMEs) hold a smaller share but are expected to grow as affordable cloud solutions become more accessible.
- Biopharmaceutical companies emerged as the top end-users in 2023, accounting for 42.3% of the total market revenue.
- Their dominance is fueled by high production complexity, strict regulations, and the need for robust digital process management.
- North America led the global market in 2023, securing a significant 40.7% share, thanks to strong technological infrastructure and high R&D investments.
- The region’s dominance is also supported by a concentration of major pharmaceutical manufacturers and favorable government policies.
Regional Analysis
North America leads the pharmaceutical manufacturing software market with a 40.7% revenue share. This dominance is driven by technological advancements and a growing need for efficient drug production. Digital transformation is helping pharmaceutical firms improve compliance and reduce costs. A notable example is the 2021 partnership between Amazon Web Services (AWS) and Pfizer. Their goal is to create cloud-based solutions that improve drug development and clinical trials. Companies in the region are adopting AI, big data, and automation to optimize operations and speed up product launches.
Advanced software tools are now key to maintaining quality control and ensuring regulatory compliance in North America. These tools help pharmaceutical companies streamline operations and meet complex supply chain demands. The region is also facing increased regulatory pressure, prompting firms to improve traceability and transparency. As a result, pharmaceutical manufacturers are relying more on software to manage production and distribution efficiently. This trend is expected to continue, supporting sustained market growth and encouraging further investment in digital manufacturing platforms.
Asia Pacific is projected to witness the highest growth rate in this market. Countries like China and India are at the forefront, modernizing pharmaceutical facilities through digital adoption. A major example is the 2022 partnership between Triastek, Inc. and Siemens Ltd., aimed at boosting digital manufacturing using 3D printing and automation. Such collaborations reflect the region’s focus on innovation and compliance. Backed by government support and rising healthcare demand, Asia Pacific’s pharmaceutical software market is set for strong and steady expansion during the forecast period.
Segmentation Analysis
Deployment Type Analysis
In 2023, the cloud deployment segment led the pharmaceutical manufacturing software market with a 72.5% share. More pharmaceutical companies are adopting cloud-based solutions for better scalability, flexibility, and cost-efficiency. These solutions enable real-time data access, production optimization, and improved team collaboration across locations. The shift to digital transformation and the need for seamless integration with existing systems are driving this trend. Cloud software also boosts regulatory compliance and data security. As agility becomes vital, cloud adoption is expected to grow across all pharmaceutical manufacturing segments.
Application Analysis
Large enterprises dominated the application segment with a 67.9% share in 2023. This is due to the growing operational complexity faced by big pharmaceutical firms. These organizations need advanced software to manage compliance, efficiency, and vast amounts of data. Many are investing in integrated systems to automate supply chain tasks, enhance quality control, and improve traceability. The rising use of AI and IoT by large enterprises is further pushing software adoption. These technologies support smarter decision-making and drive improvements in production efficiency and regulatory adherence.
End-user Analysis
Biopharmaceutical companies accounted for 42.3% of the market, showing strong growth. This is driven by the rising demand for biologics and the complex manufacturing processes involved. These companies use software to manage batch production, raw material tracking, and regulatory compliance. Personalized medicine and biologic therapies are also fueling software needs. Biopharma firms face unique challenges in ensuring product safety and consistency. As the sector expands, the demand for specialized manufacturing software will grow. These tools will be key to achieving compliant and efficient production processes in the evolving biopharma landscape.
By Deployment Type
- On-premise
- cloud
By Application
- Large Enterprises
- SMEs
By End-User
- Medical Device Companies
- Biopharmaceutical Companies
- Academic Research Institutions
- Contract Research Organizations
- Others
Key Players Analysis
The pharmaceutical manufacturing software market is highly competitive, driven by innovation and tech integration. Key players focus on adopting automation, data analytics, and cloud-based systems to boost efficiency. These technologies help optimize production, ensure quality, and meet compliance standards. Strategic partnerships are also essential. Companies team up with pharmaceutical firms and contract manufacturers to expand their reach. These alliances allow them to co-develop better solutions and tap into new markets. The growing demand for scalable and user-friendly platforms further shapes this dynamic sector.
Dassault Systèmes stands out as a major player in this market. The company offers advanced software solutions through its “BIOVIA” suite. This platform uses AI, big data, and cloud technology to streamline drug development and manufacturing. Dassault helps pharma companies cut costs, speed up production, and stay compliant with global regulations. It also builds custom solutions in partnership with clients. This co-development strategy improves adoption rates and strengthens its market position through innovation and collaboration.
Top Key Players in the Pharmaceutical Manufacturing Software Market
- Wipro
- TATA
- Dassault Systemes
- IBM
- Honeywell
- Cognizant
- Capgemini
- Apple
- Accenture
Emerging Trends
1. Shift Toward Cloud-Based Platforms
Pharmaceutical companies are moving away from traditional on-site systems. They’re switching to cloud-based platforms for more flexibility. With cloud software, teams can access manufacturing data from anywhere, at any time. This improves collaboration across departments and speeds up decision-making. Cloud systems also help reduce IT maintenance costs. Updates are automatic, and scalability is easier. As a result, companies don’t need to invest heavily in hardware or local servers. The cloud also supports remote work and global operations. This shift is especially useful for large pharmaceutical companies and contract manufacturers working across borders.
2. Integration with Automation and IoT
There is a growing trend of combining software with automation and IoT devices. These systems help monitor manufacturing in real time. Sensors and connected machines give instant feedback on performance and quality. This allows for quick responses to problems and reduces human error. It also improves safety and compliance. Automated alerts and predictive maintenance are becoming more common. The goal is to make the production line smarter and more efficient. Integrating software with smart equipment leads to better control, fewer breakdowns, and higher output in pharmaceutical manufacturing.
3. Greater Focus on Regulatory Compliance
Regulatory compliance is a top priority for pharmaceutical companies. New software solutions now include features that support this need. These tools offer electronic batch records, automated audit trails, and digital signatures. All of these are crucial for meeting FDA and global standards. Built-in compliance tools help avoid errors and reduce the risk of fines or recalls. They also speed up inspections and audits. As regulations grow more complex, having software that simplifies compliance is essential. Companies are investing in platforms that help them maintain quality and safety at every step.
4. Adoption of Artificial Intelligence and Data Analytics
AI and data analytics are transforming pharmaceutical manufacturing. Companies now use predictive analytics to spot issues before they happen. This helps reduce downtime and improve equipment performance. AI also supports better scheduling and inventory planning. These tools analyze large data sets quickly, revealing patterns that humans might miss. The result is smarter decision-making and more efficient operations. Data analytics can also improve product quality and consistency. With AI, companies can move from reactive to proactive manufacturing strategies, saving time and money while boosting output.
5. Customization and Scalability
Software providers now offer more flexible, customizable solutions. These platforms can be tailored to fit the unique needs of different businesses. Whether it’s a small biotech startup or a large pharmaceutical company, the software can scale as operations grow. Custom modules can be added for specific tasks like quality control or inventory management. This means companies only pay for what they need. Scalability ensures the software stays useful over time. Customization helps businesses align their digital tools with actual workflows. This approach boosts productivity and supports long-term growth.
6. Focus on End-to-End Supply Chain Visibility
Modern manufacturing software is going beyond the factory floor. It’s now helping companies manage the full supply chain. This includes tracking raw materials, managing supplier relationships, and monitoring shipments. With real-time updates, companies can respond faster to disruptions or delays. This improves planning and helps avoid shortages or overstock. End-to-end visibility also boosts accountability and quality control. By integrating supply chain data into one system, pharmaceutical firms gain a clearer view of operations. This transparency leads to smarter decisions and a more resilient manufacturing process.
Use Cases
1. Production Planning and Scheduling
Pharmaceutical companies use software to plan and schedule their manufacturing activities. This ensures that each step—from mixing to packaging—happens at the right time and in the correct order. With better scheduling, delays are reduced and production runs more smoothly. The software also helps allocate resources efficiently. It ensures that workers, machines, and materials are available when needed. This cuts down on downtime and reduces waste. Companies can also quickly adjust their plans when demand changes or an issue arises. As a result, production becomes more flexible, faster, and cost-effective.
2. Quality Control and Batch Management
Quality is critical in the pharmaceutical industry. Manufacturing software helps track every batch from start to finish. Each batch gets a unique ID and full traceability. If there’s a problem, the company can find the source fast—whether it’s a faulty ingredient or a missed step. This helps fix issues quickly and protects patients from unsafe products. The software also stores quality test results and inspection reports. It flags anything that falls outside set standards. This ensures high-quality medicines, reduces recalls, and keeps companies in line with strict health regulations.
3. Regulatory Reporting and Documentation
Pharmaceutical software simplifies compliance. It automatically generates and stores all required records. This includes temperature logs, cleaning records, operator actions, and more. Regulatory agencies require detailed documentation, and this software keeps it ready for inspection. Reports are created in real time, reducing the need for manual data entry. This saves time and cuts down on human errors. It also helps companies respond quickly to audits. With accurate and complete records, manufacturers stay compliant and avoid penalties. The software keeps all data safe and easy to retrieve when needed.
4. Inventory and Material Tracking
Managing inventory is easier with pharmaceutical manufacturing software. The system tracks raw materials, components, and finished products in real time. It alerts users when stock is low or when items are close to expiring. This helps avoid both shortages and overstocking. The software also ensures that materials are used in the right order. This reduces waste and keeps production moving. With full visibility of inventory, companies can plan better and cut costs. The software also supports barcode scanning and lot tracking. This adds another layer of control and traceability.
5. Equipment Maintenance and Monitoring
Machine breakdowns can cause costly delays. To avoid this, companies use software to schedule regular maintenance. The system tracks each piece of equipment and alerts staff before issues arise. It also monitors machine performance in real time. This helps identify early signs of wear or failure. Scheduled maintenance reduces downtime and extends machine life. The software logs all maintenance activities for future audits. It also helps plan spare parts inventory. With better machine health, production stays on track, and the risk of unexpected stops is lower.
6. Electronic Batch Records (EBR)
Electronic Batch Records (EBR) are essential for modern pharma production. This software creates digital records for each batch. These records capture every step—from material input to final packaging. EBR ensures that all steps are followed and documented properly. It helps reduce human error and improves compliance. The system also provides real-time visibility into batch status. If a deviation occurs, it can be caught and corrected quickly. EBR is audit-ready, making inspections faster and easier. It replaces paper-based systems, which are harder to manage and easier to lose or damage.
7. Collaboration Across Departments
Pharmaceutical software connects different departments through one system. It gives production, quality control, and regulatory teams access to the same data. This improves coordination and speeds up decisions. Dashboards show real-time updates on inventory, production status, and quality checks. Teams can work together more easily, even if they are in different locations. This reduces delays and miscommunication. With everyone on the same page, it’s easier to manage complex processes. The result is smoother operations, faster problem-solving, and better overall efficiency in drug manufacturing.
Conclusion
In conclusion, the pharmaceutical manufacturing software market is growing steadily as companies aim to improve efficiency, quality, and compliance. The shift to cloud-based platforms, along with the use of AI and automation, is helping drug manufacturers modernize their operations. As regulations become more strict, software tools are proving essential for maintaining accurate records and streamlining audits. Both large enterprises and growing biopharmaceutical firms are adopting these solutions to stay competitive. With strong demand across regions like North America and Asia Pacific, the market is expected to keep expanding. As digital transformation continues, software will play a key role in shaping the future of pharmaceutical manufacturing.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



