Table of Contents
Overview
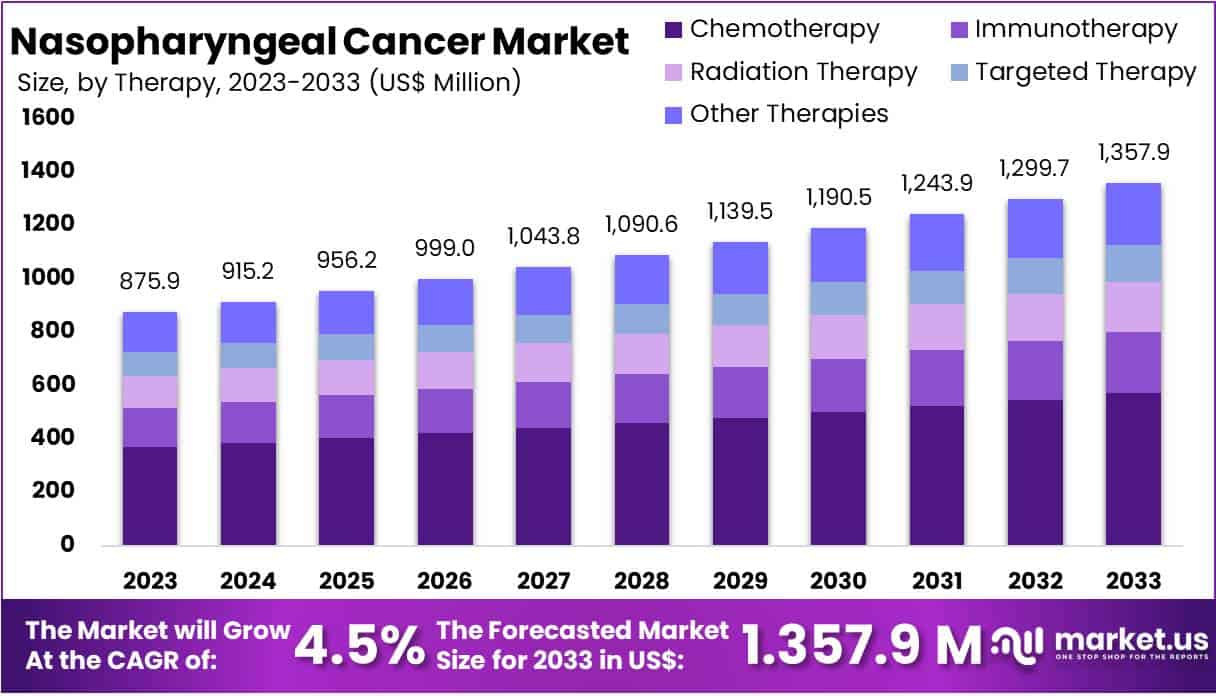
New York, NY – July 23, 2025 – The Global Nasopharyngeal Cancer Market size is expected to be worth around US$ 1357.9 Million by 2033, from US$ 875.9 Million in 2023, growing at a CAGR of 4.5% during the forecast period from 2024 to 2033. North America dominated the market, securing a 46.1% share with a market value of US$ 403.8 million.
Nasopharyngeal cancer (NPC) is a rare type of head and neck cancer that begins in the nasopharynx, the upper part of the throat behind the nose. While uncommon globally, it is more prevalent in certain regions, particularly Southeast Asia, North Africa, and the Middle East.
The formation of NPC is strongly associated with genetic predisposition, Epstein-Barr Virus (EBV) infection, and environmental factors such as high consumption of preserved foods containing nitrosamines. Other risk factors include smoking, heavy alcohol use, and prolonged exposure to wood dust and formaldehyde.
NPC often develops without early symptoms, making diagnosis challenging. When symptoms do appear, they may include a persistent lump in the neck, nasal obstruction or bleeding, hearing loss (often in one ear), and frequent headaches. Because of its location, the tumor may also affect nearby nerves and lead to facial numbness or visual changes.
Diagnosis typically involves a combination of nasoendoscopy, biopsy, MRI or CT scans, and blood tests for EBV DNA levels. Early detection plays a critical role in improving outcomes, as NPC tends to respond well to radiation therapy and chemotherapy.
Awareness and education are key to recognizing risk factors and symptoms early. Ongoing research continues to explore targeted therapies and immunotherapies to improve survival and quality of life for patients with nasopharyngeal cancer.
Key Takeaways
- The global nasopharyngeal cancer market is anticipated to grow from USD 875.9 million in 2023 to USD 1,357.9 million by 2033, reflecting a compound annual growth rate (CAGR) of 4.5% during the forecast period.
- North America emerged as the leading regional market in 2023, accounting for 46.1% of the global share, with an estimated valuation of USD 403.8 million. This dominance is primarily attributed to the region’s well-established healthcare infrastructure and strong investment in cancer research and treatment.
- Chemotherapy remains the most utilized treatment approach, comprising over 42.1% of the therapy segment in 2023. Its continued prevalence is driven by its effectiveness in managing advanced-stage nasopharyngeal carcinoma.
- Hospitals and clinics represent the largest end-user group, capturing more than 41.7% of the total market share in 2023. Their significant role is linked to the availability of specialized oncology departments, advanced diagnostic tools, and comprehensive care facilities for cancer patients.
Segmentation Analysis
- Therapy Analysis: In 2023, chemotherapy led the nasopharyngeal cancer therapy market with over 42.1% share, attributed to its efficacy in treating advanced-stage disease. It remains the first-line option for tumor reduction and survival improvement. Immunotherapy is gaining traction due to its targeted mechanism and fewer side effects, while radiation therapy continues to be essential in early-stage management. Targeted therapies and experimental approaches like gene and hormonal therapy are showing promise, expanding the treatment landscape through precision and innovation.
- End-Users Analysis: Hospitals and clinics dominated the end-user segment in 2023, holding more than 41.7% of the market share. Their leadership stems from the availability of advanced diagnostic tools, comprehensive treatment modalities, and multidisciplinary care teams. These settings support chemotherapy, radiation, and targeted therapies under one roof. Strong infrastructure investments and evolving clinical capabilities enable these institutions to deliver high-quality, personalized care. As new technologies emerge, hospitals and clinics are expected to retain their key role in nasopharyngeal cancer management.
Market Segments
By Therapy
- Chemotherapy
- Immunotherapy
- Radiation Therapy
- Targeted Therapy
- Other Therapies
By End-Users
- Hospitals and Clinics
- Ambulatory Surgery Centers
- Cancer Research Institutes
- Other End-Users
Regional Analysis
In 2023, North America held a dominant position in the global nasopharyngeal cancer market, accounting for over 46.1% of the total market share, with a valuation of approximately USD 403.8 million. This leadership is primarily supported by the region’s advanced healthcare infrastructure, which facilitates early diagnosis and access to cutting-edge treatment modalities.
Widespread awareness of nasopharyngeal cancer symptoms contributes to prompt medical intervention, while high levels of investment in research and development by academic and clinical institutions continue to accelerate therapeutic innovation. The presence of leading oncology research centers in the United States and Canada enables rapid adoption of novel treatments, significantly improving patient outcomes.
Government-supported healthcare policies promote regular screenings and allocate substantial funding for cancer research initiatives. These efforts reinforce North America’s capacity to support both conventional and emerging treatment approaches.
Additionally, comprehensive insurance coverage ensures broader patient access to advanced cancer therapies. This financial accessibility plays a vital role in maintaining high treatment uptake across the region.
Looking ahead, the nasopharyngeal cancer market in North America is expected to expand steadily, driven by ongoing innovation, favorable regulatory frameworks, and sustained public health investment. The region is projected to retain its leadership in both market value and therapeutic advancements throughout the forecast period.
Emerging Trends
- Integration of Immunotherapy into Standard Care: It has been observed that immune checkpoint inhibitors targeting PD 1 are now being added to chemotherapy for recurrent or metastatic nasopharyngeal carcinoma (NPC). In a pivotal trial (JUPITER 02), median progression free survival increased from 8.0 months with chemotherapy alone to 11.7 months when toripalimab was added, and two year overall survival rose from 65% to 78%. Following FDA approval in January 2024, toripalimab became the first immunotherapy specifically approved for NPC, marking a shift toward biologic agents in this disease.
- Liquid Biopsy via EBV DNA Screening: The implementation of circulating Epstein Barr virus (EBV) DNA tests has been shown to enable earlier detection of NPC. In a recent study, an EBV DNA assay demonstrated a sensitivity of 97.1% (95% CI, 95.5%–98.7%) and specificity of 98.6% (95% CI, 98.6%–98.7%) for NPC screening. Screen detected cases were more often diagnosed at stage I–II (71% vs. 20% historically), suggesting lead time advantages for early stage identification.
- De‑escalated Radiation Guided by Treatment Response: It has been noted that clinical trials are exploring induction chemotherapy–guided intensity modulated radiation therapy (IMRT) protocols to spare normal tissues. Such response guided approaches aim to reduce long term toxicities (e.g., xerostomia, dysphagia) by tailoring radiation dose intensity according to tumor shrinkage after initial chemotherapy.
- Advances in Precision Imaging and AI Tools: The development of advanced imaging modalities (e.g., functional MRI, PET) combined with emerging AI driven analytics is being applied to better delineate tumor margins and predict treatment response. Early stage studies by NIH researchers have demonstrated AI models capable of forecasting immunotherapy benefit, laying groundwork for personalized NPC care.
Use Cases
- Community Based EBV DNA Screening: In a large demonstration project in southern China, approximately 52,000 men and women aged 30–69 were enrolled in an EBV based antibody and DNA screening arm. Of these, 1,112 individuals (1.5% of all participants) tested positive for EBV DNA in plasma; 309 of them (27.8% of positives) had persistently detectable levels. NPC was confirmed in 34 participants (11.0% of the persistently positive group) after endoscopy, MRI, and biopsy.
- First Line Treatment of Recurrent/Metastatic NPC: Toripalimab plus chemotherapy was used as an initial treatment for recurrent or metastatic NPC. In the POLARIS 02 trial, toripalimab monotherapy achieved an objective tumor shrinkage rate of 21%, with a median duration of response of nearly 15 months.
- Maintenance Immunotherapy to Prolong Remission: After up to six months of combined therapy, toripalimab was continued as maintenance until disease progression. Final analyses reported median progression‑free survival of 21.4 months versus 8.2 months for chemotherapy alone, and three‑year overall survival of 64% versus 49%.
- Response Adapted Radiotherapy: Response guided IMRT is being tested to tailor radiation doses based on chemotherapy response, with the goal of reducing grade 3–4 side effects. Although detailed outcome data are pending publication, this approach exemplifies the move toward adaptive radiotherapy protocols in NPC care.
Conclusion
The global nasopharyngeal cancer market is witnessing steady growth, driven by advancements in diagnostics, therapies, and regional healthcare investments. Chemotherapy remains the primary treatment, while immunotherapy and precision-guided radiotherapy are transforming clinical approaches. North America leads the market due to superior infrastructure and research funding.
Innovations such as EBV DNA screening and AI-enabled imaging are enhancing early detection and personalized care. Clinical trials continue to validate the efficacy of emerging therapies like toripalimab, signaling a shift toward biologics. As technology and treatment paradigms evolve, the market is expected to expand further, improving survival outcomes and quality of life for patients.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



