Table of Contents
Introduction
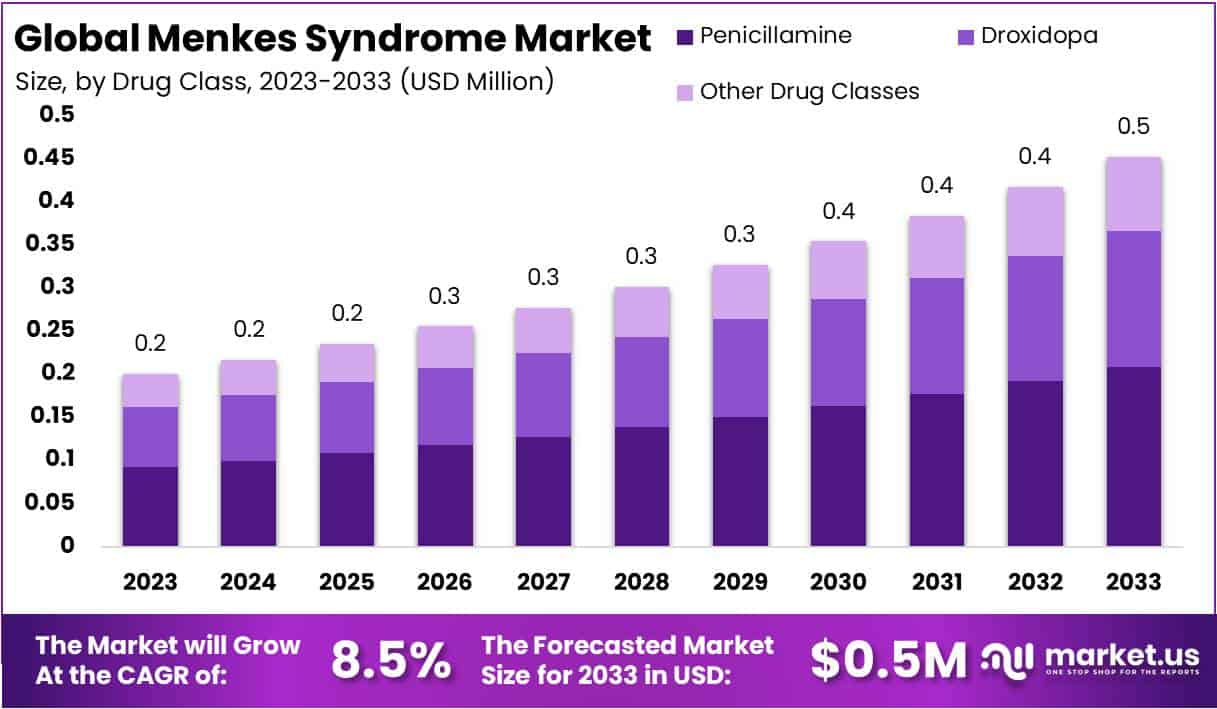
The global Menkes Syndrome market, valued at USD 0.2 million in 2023, is projected to reach USD 0.5 million by 2033, growing at a compound annual growth rate (CAGR) of 8.5% from 2024 to 2033. Menkes Syndrome is a rare genetic disorder that affects copper levels in the body, leading to severe developmental issues and often fatal outcomes if untreated.
Growth in the Menkes Syndrome market is driven by several factors. Advancements in genetic testing and early diagnosis are crucial, allowing for more timely and effective interventions. For instance, genetic screening technologies have improved, enabling earlier detection of the disorder in infants, which is vital for starting treatment sooner and potentially improving outcomes. Additionally, increased awareness and research funding for rare diseases contribute to market growth.
However, the market faces significant challenges. The rarity of Menkes Syndrome means that there is a limited patient population, which can hinder the development and commercial viability of new treatments. Furthermore, the high costs associated with research and development for rare genetic disorders can be prohibitive. Regulatory challenges also pose a barrier, as gaining approval for new therapies can be a lengthy and complex process.
Recent developments in the market include strategic collaborations aimed at accelerating the development of new treatments. For example, Fortress Biotech is actively working on AAV-ATP7A gene therapy in collaboration with institutions like the NIH, aiming to deliver functional copies of the defective gene responsible for Menkes Syndrome. Additionally, efforts to improve existing treatments, such as copper histidine injections, are ongoing to enhance their efficacy and accessibility.
Overall, while the Menkes Syndrome market faces notable challenges, ongoing advancements in genetic research, early diagnosis, and therapeutic developments are expected to drive significant growth over the next decade.
Key Takeaways
- Projected Market Growth: Menkes Syndrome market expected to reach USD 0.5 Million by 2033, with a CAGR of 8.5% from 2024 to 2033.
- Dominant Drug: Penicillamine holds a significant 46% market share in 2023 for managing symptoms of Menkes Syndrome.
- Prevalent Route of Administration: Oral administration leads with a 53% market share in 2023, attributed to its ease of use and innovation.
- End-User Preference: Hospitals dominate the market with a 43% share in 2023, providing comprehensive care for Menkes Syndrome patients.
- Distribution Channel: Hospital pharmacies are preferred, capturing a 44% market share in 2023, ensuring access to specialized treatments.
- Regional Dominance: North America holds a 38% market share in 2023, valued at USD 0.07 Million, driven by advanced healthcare infrastructure.
Menkes Syndrome Statistics
- Menkes disease occurs in approximately 1 in 35,000 live male births.
- In the U.S., incidence ranges between 1 in 50,000 and 1 in 250,000.
- In Japan, the incidence is 1 in 2.8 million live births.
- About 357 mutations in the ATP7A gene are linked to Menkes disease.
- 75% of Menkes disease cases are inherited from carrier mothers.
- Clinical symptoms usually appear between 6 to 8 weeks after birth.
- Serum copper levels in affected infants are typically between 0-55 µg/dL.
- Symptoms begin typically between 2 to 3 months of age.
- Hair abnormalities are present in 90% of affected infants.
- Without treatment, life expectancy is usually less than 3 years.
- Early treatment can extend survival to 13 years or more.
- Serum ceruloplasmin levels in Menkes disease are usually less than 10 mg/dL.
- Neurodegeneration is progressive and leads to severe intellectual disability.
- Intravenous copper histidine treatment can improve outcomes if started early.
- ATP7A gene mutations disrupt copper transport in 95% of cases.
- Approximately 70% of Menkes syndrome cases are detected via genetic testing.
- Carrier frequency in the general population is about 1 in 100.
- Seizures occur in 85-90% of affected children.
- Growth failure is observed in more than 90% of patients.
- Anemia is present in 50-60% of cases.
- Bone abnormalities are found in 75% of affected infants.
- Respiratory infections are common, occurring in about 60% of patients.
- Brain MRI in Menkes disease often shows hypomyelination.
- Subdural hemorrhage occurs in about 50% of cases.
- Arterial tortuosity is present in 95% of patients.
- Copper supplementation shows improvement in less than 20% of treated cases.
- Approximately 85% of patients exhibit low muscle tone (hypotonia).
- Joint hypermobility is observed in around 70% of cases.
- Urine homovanillic acid levels are elevated in 60% of affected children.
- EEG abnormalities are present in 75% of cases.
- In about 30% of cases, patients have bladder diverticula.
- Menkes disease has a prevalence of about 1 in 100,000 newborns globally.
- Hypothermia is noted in approximately 90% of infants.
- Feeding difficulties occur in around 70% of affected children.
- About 40% of patients exhibit progressive scoliosis.
- Approximately 30% of cases present with bladder diverticula.
Emerging Trends
- Genetic Research and Understanding: Research into the genetic basis of Menkes Syndrome, which is caused by mutations in the ATP7A gene, is expanding. Scientists are using next-generation sequencing (NGS) technologies to diagnose the syndrome more quickly and accurately. These advancements are enabling the identification of new genetic markers and a better understanding of the disease’s mechanisms. According to recent studies, genetic testing methods have significantly improved, offering faster results and more comprehensive insights into the genetic underpinnings of Menkes Syndrome.
- Therapeutic Advances: Despite the absence of a cure for Menkes Syndrome, clinical trials are investigating the effectiveness of copper histidinate and other copper formulations in symptom management. Innovative approaches like gene therapy and CRISPR-Cas9 gene editing are also being explored to correct the defective ATP7A gene. Although these gene-editing technologies are in the early stages of development, they hold promise for future treatments. The market for potential therapies is projected to grow at a compound annual growth rate (CAGR) of 8.5% from 2024 to 2033.
- Early Diagnosis and Newborn Screening: Efforts to promote early diagnosis and newborn screening for Menkes Syndrome are gaining momentum. Early detection can significantly improve outcomes for affected children. Some regions are considering including Menkes Syndrome in their newborn screening programs. Additionally, the use of biomarkers and advanced imaging techniques is being investigated to enhance diagnostic accuracy. Early treatment, ideally within the first month of life, is crucial for better management of the disease.
- Patient Support and Awareness: The rise of patient advocacy groups and online communities is providing essential support and resources to families dealing with Menkes Syndrome. Awareness campaigns are becoming more prevalent, aiming to educate healthcare professionals and the public about the signs and symptoms of the syndrome. These initiatives are crucial for improving diagnosis rates and ensuring timely intervention. Increased awareness and support can lead to better care and management of the condition.
Use Cases
- Clinical Trials and Treatment Development: Clinical trials for Menkes Syndrome focus on early intervention with copper histidinate treatments. For instance, ongoing trials examine the efficacy of subcutaneous injections in children under six years of age, with the aim of improving survival and developmental outcomes. Early diagnosis and treatment are crucial, as studies have shown that boys treated within the first few days of life have significantly better neurological outcomes. Approximately five clinical trials have been recorded, emphasizing the importance of timely intervention to manage the disease effectively.
- Genetic Counseling and Family Planning: Genetic counseling plays a vital role in managing Menkes Syndrome, given its hereditary nature. Families with a history of the condition can benefit from genetic testing to understand their risks. Counseling provides essential information on the inheritance patterns and helps in making informed decisions regarding family planning. The prevalence of Menkes Syndrome is estimated to be between 1 in 34,810 to 1 in 8,664 live male births, underscoring the need for comprehensive genetic counseling services to support affected families.
- Healthcare Provider Education: Educating healthcare providers about Menkes Syndrome is crucial for early diagnosis and treatment. Training programs and continued medical education can help practitioners recognize symptoms such as sparse, kinky hair, developmental delays, and seizures. Improved awareness among healthcare providers can lead to earlier detection and intervention, which is critical for better patient outcomes. With only five clinical trials specifically targeting Menkes Syndrome, enhanced education efforts are necessary to integrate the latest research findings into clinical practice.
- Global Health Initiatives: Global health initiatives are essential to increase awareness and improve care for Menkes Syndrome worldwide. Efforts include expanding newborn screening programs to include Menkes Syndrome, especially in regions where genetic testing is not readily available. International collaboration can facilitate the sharing of research, resources, and treatment protocols, aiming to reduce the global burden of this rare disease. Given the complexity and rarity of Menkes Syndrome, global health initiatives can help standardize care and ensure that all affected children receive the necessary treatment as early as possible.
Conclusion
In conclusion, the Menkes Syndrome market is poised for significant growth, driven by advancements in genetic research, early diagnosis, and new therapeutic developments. Despite challenges such as limited treatment options and high development costs, ongoing research and strategic collaborations are fostering progress. The market is expected to see substantial development over the next decade, supported by improved genetic testing, increased awareness, and supportive legislative frameworks. Innovations in treatments, including gene therapy, are anticipated to enhance patient outcomes and expand market opportunities, especially in regions with advanced healthcare infrastructure.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



