Table of Contents
Introduction
Global Medical Device Cleaning Market size is expected to be worth around USD 86.7 billion by 2032 from USD 17.9 billion in 2023, growing at a CAGR of 17.60% during the forecast period from 2024 to 2032.
The medical device cleaning market is poised for growth, driven by the high demand for surgical procedures and the rising prevalence of chronic diseases and other medical conditions. The necessity for frequent cleaning and sterilization of surgical tools and medical devices is critical to ensuring patient safety and optimal clinical outcomes. The increase in healthcare-associated infections (HAIs) has further highlighted the need for stringent cleaning practices.
Healthcare institutions primarily handle the cleaning and disinfection of medical equipment, a practice that is vital in preventing severe health complications, prolonged hospital stays, and increased healthcare costs. The emphasis on proper cleaning practices is gaining importance among both healthcare professionals and patients, fostering a culture of safety and sanitation within healthcare facilities, thereby enhancing the demand for cleaning products.
The COVID-19 pandemic is expected to positively impact the market. In healthcare surrounding, where medical procedures are performed, surfaces are at a high risk of contamination with the COVID-19 virus. It is crucial that these surfaces are thoroughly cleaned and disinfected to prevent the virus’s transmission, particularly in areas admitting COVID-19 patients. This necessity extends to non-traditional isolation venues for patients with uncomplicated and moderate COVID-19, such as homes and alternative facilities, potentially driving market growth.
Additionally, diabetic patients, who are at an increased risk of developing urinary tract infections (UTIs), require consistent and effective infection control measures. This clinical need to prevent site infections and the use of personal protective equipment contributes to the rising demand in the medical device cleaning market. Furthermore, the growing incidence of surgical site infections is expected to further boost market expansion.
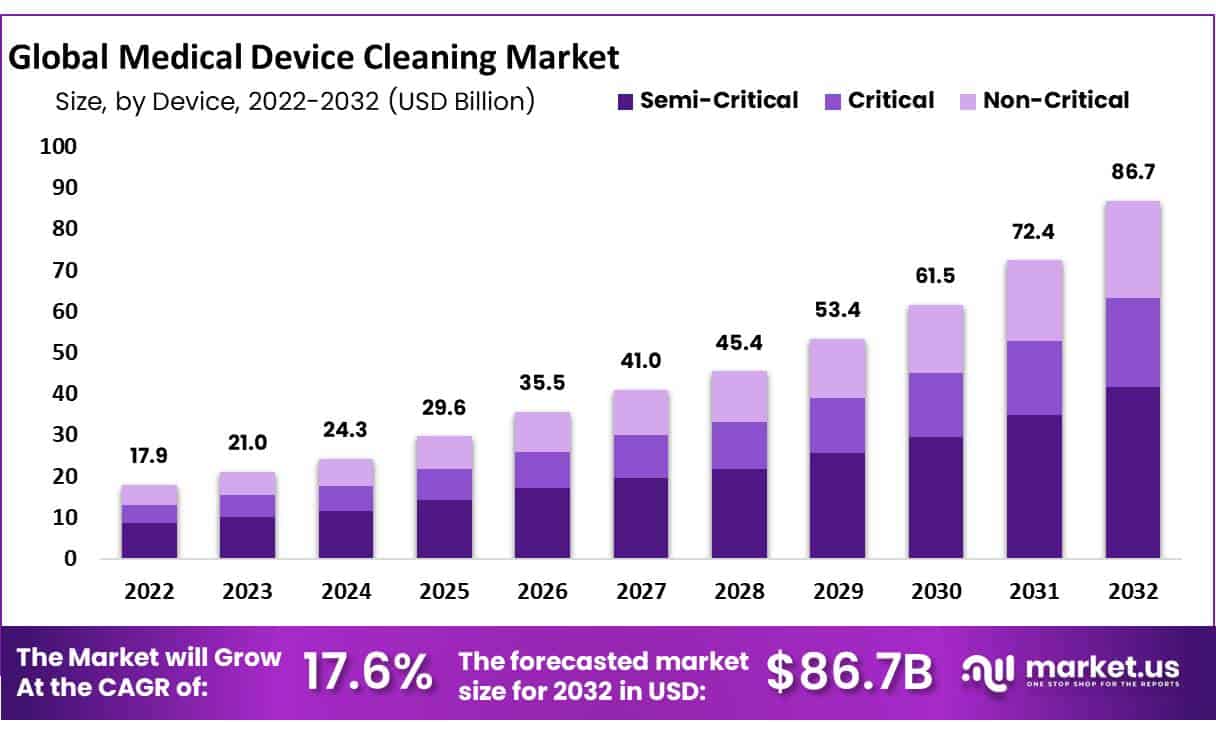
Key Takeaways
- Market Size: Medical Device Cleaning Market size is expected to be worth around USD 86.7 billion by 2032 from USD 17.9 billion in 2023.
- Market Growth: The market growing at a CAGR of 17.60% during the forecast period from 2024 to 2032.
- Process Analysis: The Disinfection Segment Dominated the Medical Device Cleaning Market.
- EPA Analysis: The intermediate-level segment dominated the medical device cleaning market and held a lucrative market share of 52% in 2022.
- Application Analysis: The Surgical Instruments Segment Held the Largest Revenue Share in 2022
- End-Use Analysis: Hospitals & Clinics Segment Estimated to Contribute Significantly to the Market Growth
- Regional Analysis: North America accounted for a significant medical device cleaning market revenue share of 34.6%.
Medical Device Cleaning Statistics
- Adherence to Guidelines and Training Outcomes:
- Approximately 80% of health facilities now adopt WHO guidelines for the reprocessing of medical devices.
- Observational studies show 70% compliance with proper decontamination practices in health institutions.
- Post-training improvements have led to a 60% increase in health worker adherence to disinfection protocols.
- Impact on Infections and Contamination:
- A 30% reduction in device-related infections has been observed with strict adherence to WHO cleaning protocols.
- Around 50% of medical devices show signs of contamination when cleaning guidelines are not strictly followed.
- Resource Availability and Maintenance Challenges:
- Only 10% of low-income facilities have access to advanced cleaning equipment, highlighting resource limitations.
- 40% of hospitals report maintenance challenges directly related to cleaning practices.
- Cleaning Practices and Device Longevity:
- 15% of reusable devices experience deterioration due to the use of improper cleaning agents.
- The effectiveness of sterilization reaches up to 90% when WHO guidelines are properly implemented.
- There is a 20% higher risk of cross-contamination associated with inadequate drying of devices.
- 35% reduction in device longevity has been noted when harsh cleaning chemicals are used.
- Training, Compliance, and Automation:
- Despite recommendations, 25% of devices are cleaned more frequently than suggested by manufacturers, which may not necessarily equate to safer practices.
- 85% compliance with hand hygiene is reported during device cleaning processes, indicating relatively high adherence to this particular protocol.
- A significant portion, 45% of healthcare providers, indicates a need for additional training on proper device cleaning.
- 55% of healthcare facilities have implemented automated cleaning systems, though 70% of devices still require manual intervention.
- Challenges in Practice:
- Time constraints are cited as the primary reason for 60% of reported non-compliance with cleaning protocols.
- Early disposal issues are noted, with 5% of medical devices being discarded prematurely due to damage from improper cleaning.
Medical Device Cleaning Process Analysis
- Sterilization: Sterilization involves the complete destruction of all microbial life, including viruses, bacteria, and spores, on medical devices. This is achieved through methods such as steam under pressure (autoclaving), ethylene oxide gas, hydrogen peroxide gas, plasma, ozone, and radiation. The choice of method often depends on the device’s material and sensitivity to heat and moisture. Sterilization is critical for devices that enter sterile parts of the body or have contact with the bloodstream.
- Disinfection: Disinfection reduces the number of pathogenic microorganisms on surfaces or objects to levels considered safe for public health. Unlike sterilization, disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores. It involves the use of chemical agents such as alcohols, chlorine, and aldehydes or physical methods like heat to destroy pathogens that could cause infection. This process is used for medical devices that contact mucous membranes but do not penetrate sterile tissues.
- Ethylene Oxide (EtO) Sterilization: Ethylene oxide is a chemical sterilization method used extensively for heat-sensitive medical devices. It is highly effective in penetrating the surfaces of complex devices and killing all known viruses, bacteria, and fungi. However, EtO must be handled carefully due to its highly flammable and potentially carcinogenic nature. This method is suited for materials that cannot tolerate the high temperatures of steam sterilization.
- Cleaning: Cleaning is the physical removal of organic and inorganic material such as blood, soil, and other contaminants from devices. It typically involves the use of water with detergents or enzymatic cleaners and is a crucial first step before high-level disinfection or sterilization can occur. Effective cleaning is necessary to reduce the microbial load and prevent the risk of device-related infections.
- Automatic Cleaning: Automatic cleaning involves the use of automated equipment such as washers and disinfectors, which standardize the cleaning process by controlling variables like time, temperature, and chemical exposure. These systems are especially useful in settings that require high throughput and consistency, such as hospitals and clinical labs, ensuring that all devices are cleaned to the same standard.
- Manual Cleaning: Manual cleaning is a labor-intensive process that requires detailed attention to ensure all parts of the medical device are adequately cleaned. It often involves scrubbing, soaking, and rinsing with water and detergents. Manual methods are typically necessary for delicate or intricate devices that cannot withstand the rigors of automatic cleaning. This process also requires strict adherence to protocols to ensure thorough decontamination.
Emerging Trends
- Neutral pH Detergents: Neutral pH solutions with enzymatic cleaners are now widely preferred for medical devices, particularly delicate instruments, due to their material compatibility and effective soil removal.
- Enzymatic Cleaners: Cleaning solutions increasingly include enzymes like proteases for organic material removal, especially for devices exposed to protein-based substances. However, rinsing is essential to prevent potential allergic reactions in users.
- Hydrogen Peroxide Use: Low-temperature vaporized hydrogen peroxide has gained traction as a sterilization alternative, offering material compatibility for more sensitive devices, supported by recent FDA-recognized standards.
- Real-Time Cleaning Tests: While not yet widespread, real-time cleaning verification tests are being explored to enhance quality control, allowing immediate detection of residual contaminants.
- Rotational Disinfectants: Facilities are implementing rotational use of disinfectants to prevent microbial resistance, especially in high-risk environments, enhancing long-term effectiveness.
- Enhanced Inspection: Medical devices are subject to more rigorous inspections, emphasizing visual cleanliness, given that visible residues often indicate inadequate cleaning.
- Disinfection Post-Cleaning: Non-critical items and medical equipment surfaces, such as blood pressure cuffs, are disinfected regularly to control healthcare-associated infections (HAIs) due to their frequent contact with patients.
- Regulatory Compliance: FDA guidelines now include comprehensive standards for sterilization processes, including compatibility and environmental impacts, aiding manufacturers in creating safer reprocessing methods.
- Adoption of High-Level Disinfection: High-level disinfection is emphasized, especially for reusable devices like endoscopes, with guidelines outlining proper techniques to minimize infection risk.
- Single-Use Innovations: FDA supports single-use disposable components, particularly for devices that are difficult to clean effectively, reducing cross-contamination risks in complex devices.
- Duodenoscope Modifications: Reusable duodenoscopes now include disposable parts to simplify cleaning and reduce contamination risk, in response to past infection outbreaks.
- Environmental Considerations: Novel disinfection methods are being evaluated to reduce the environmental impact, such as through the safe disposal of disinfectants and more sustainable sterilization techniques.
- Collaboration in Reprocessing: Government and healthcare agencies, like the FDA and CDC, collaborate with manufacturers and healthcare facilities to improve reprocessing standards and training for medical staff.
Use Cases
- Automated Endoscope Reprocessing: Automated endoscope reprocessors (AERs) are used in hospitals to ensure consistent cleaning of endoscopes. These devices automate the complex, multi-step cleaning process, significantly reducing the risk of human error and enhancing patient safety.
- Implementation of Low-Temperature Sterilization: Certain materials in medical devices, such as flexible plastics, cannot withstand high temperatures. Using low-temperature hydrogen peroxide or ethylene oxide (EtO) sterilization methods ensures thorough cleaning without damaging the device.
- Validation of Cleaning Processes: Health facilities perform process validations on cleaning steps to ensure all equipment consistently meets cleanliness standards, especially in high-contamination risk areas, like operating rooms.
- Disposable Components for Hard-to-Clean Areas: Reusable medical devices with hard-to-clean parts, like catheters, often include disposable components. This design reduces the risk of retained biological material, improving cleaning effectiveness and patient safety.
- Neutral pH Detergents for Sensitive Instruments: For materials sensitive to acidic or alkaline solutions, hospitals use neutral pH detergents, especially in cleaning flexible and delicate devices like scopes and catheters, to avoid corrosion while ensuring cleanliness.
- Real-Time Cleaning Verification Development: Researchers are developing real-time verification systems to detect residues immediately after cleaning, aiming to improve the cleaning process through early contamination detection.
- Standardization and Staff Training in Cleaning Protocols: FDA encourages standardized procedures for device reprocessing, coupled with extensive training for healthcare staff. This standardization is particularly important in facilities where complex devices like duodenoscopes are reprocessed.
- Collaborative Cleaning Protocols for Contaminated Reusables: The CDC advises collaborative protocols for cleaning reusable devices contaminated with biological materials, emphasizing a partnership between device manufacturers, regulatory bodies, and healthcare facilities.
- Enhanced Surface Design for Ease of Cleaning: Manufacturers are designing medical devices with smooth surfaces and easily accessible parts to simplify the cleaning process, minimizing areas where bacteria and debris can accumulate.
- Microbial Testing for Cleaning Validation: Healthcare facilities conduct microbial testing on devices post-cleaning, especially in sensitive areas such as surgical rooms, to verify that cleaning processes meet regulatory standards for patient safety.
- FDA’s Sterilization Master File Program: The FDA’s voluntary Sterilization Master File program enables companies to streamline compliance by referencing existing master files for certain sterilization methods. This simplifies regulatory requirements while ensuring cleaning efficacy for approved methods like EtO sterilization.
- Use of Enzymatic Detergents for Organic Material: Enzymatic detergents are used to break down blood, tissue, and other organic materials, enhancing the cleaning process of reusable surgical instruments.
Conclusion
The medical device cleaning market is set for robust growth, driven by the increasing demand for surgical procedures and the need to control healthcare-associated infections (HAIs). The emphasis on stringent cleaning protocols has become more critical, especially in light of the COVID-19 pandemic, which has underscored the necessity for meticulous disinfection practices in healthcare settings. Advances in cleaning technologies, along with regulatory support and enhanced training protocols, are ensuring higher compliance and effectiveness in infection control. These factors collectively forecast a promising expansion in the medical device cleaning market, with a projected CAGR of 17.60% from 2024 to 2032.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



