Table of Contents
Overview
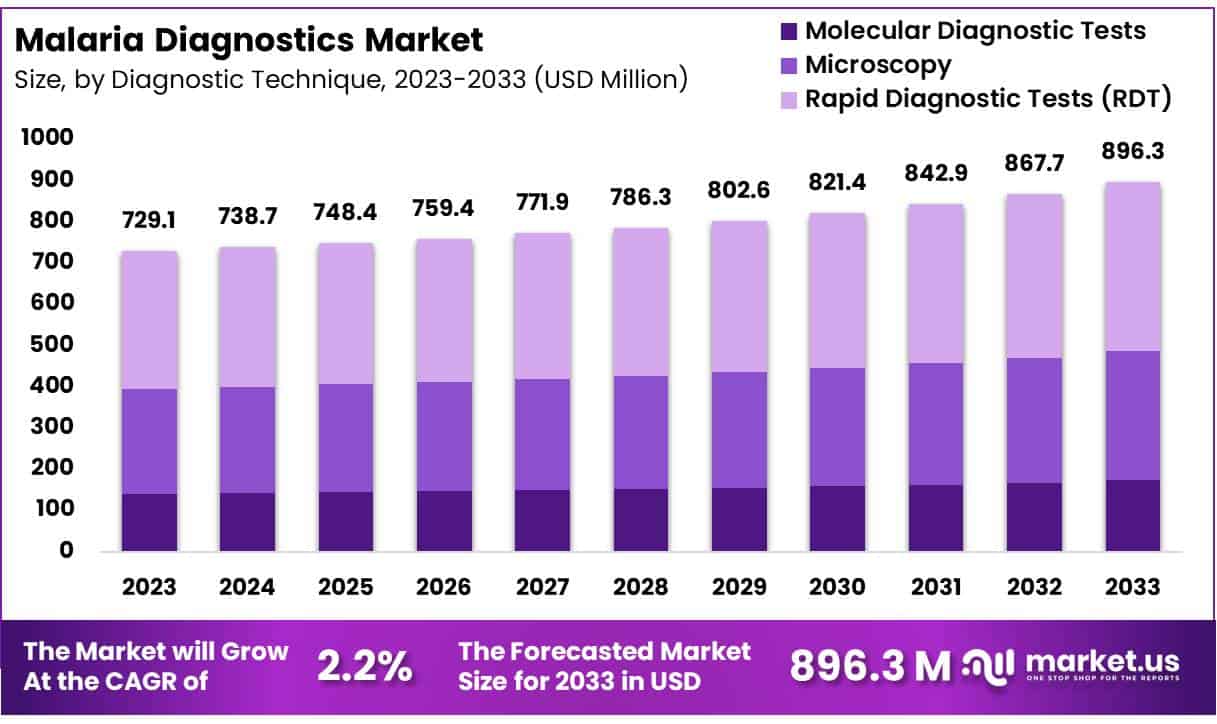
New York, NY – May 20, 2025 – Global Malaria Diagnostics Market size is expected to be worth around US$ 896.3 Billion by 2033 from US$ 729.10 Billion in 2023, growing at a CAGR of 2.2% during the forecast period from 2024 to 2033. This growth is primarily driven by the persistent global burden of malaria and the need for accurate, rapid, and accessible diagnostic solutions in endemic regions.
Malaria remains a significant public health concern, particularly in sub-Saharan Africa, Southeast Asia, and parts of South America. The increasing focus on early diagnosis and treatment, alongside global eradication efforts led by organizations such as the World Health Organization (WHO) and UNICEF, is fueling demand for diagnostic innovations.
Technological advancements in diagnostic tools, including rapid diagnostic tests (RDTs), PCR-based methods, and loop-mediated isothermal amplification (LAMP), are enhancing the accuracy and speed of malaria detection. Additionally, government-funded healthcare programs and international collaborations are supporting wider adoption in rural and low-resource settings. North America and Europe are showing moderate growth due to ongoing research and funding, while the Asia-Pacific and African regions dominate in terms of market volume, owing to high disease prevalence and expanding public health infrastructure.
Key Takeaways
- In 2023, the Malaria Diagnostics Market generated a total revenue of USD 729.10 million and is projected to reach USD 896.33 million by 2033, expanding at a compound annual growth rate (CAGR) of 2.2% over the forecast period.
- Microscopy emerged as the dominant diagnostic technique segment, accounting for the largest market share of 34.9% in terms of revenue contribution.
- On the basis of product type, Malaria Diagnostic Kits led the market, generating the highest revenue share at 52.8%.
- In terms of end-use, Hospitals and Clinics were the leading contributors, representing 44.8% of the total market share.
- Regionally, the Middle East and Africa held the dominant position in the global market, capturing a market share of 53.10%, driven by high malaria prevalence and increasing deployment of diagnostic tools in public health programs.
Segmentation Analysis
- By Diagnostic Technique Analysis: In 2023, Rapid Diagnostic Tests (RDTs) dominated the malaria diagnostics market with a 45.8% revenue share. Between 2010 and 2022, 3.9 billion RDTs were sold globally, 82% of which were distributed in sub-Saharan Africa. Their widespread use is attributed to ease of operation, fast results, and reliability in endemic areas. Meanwhile, molecular diagnostic techniques such as PCR and LAMP are gaining traction due to their high accuracy and growing importance in surveillance and precision treatment efforts.
- By Product Analysis: Malaria diagnostic kits held the largest share of the product segment in 2023, driven by their ease of use, portability, and comprehensive design that includes all necessary reagents. Their effectiveness in delivering rapid results at the point of care, especially in regions with limited infrastructure, contributes to strong adoption. Enhanced sensitivity and consistent support from public health programs and NGOs have further strengthened their role in accurate malaria detection, especially in high-burden areas.
- By End-Use Analysis: Hospitals and clinics led the end-use segment with a 44.8% share in 2023, primarily due to the growing use of RDTs for timely diagnosis and treatment. Clinics facilitate early intervention, improving patient recovery and outcomes. Meanwhile, diagnostic centers are expected to grow steadily, supported by rising patient awareness and demand for high-accuracy testing. These centers offer advanced lab capabilities and benefit from increasing government backing, including reimbursements and infrastructure investment in disease control programs.
Market Segments
By Diagnostic Technique
- Molecular Diagnostic Tests
- Conventional PCR
- Modernized PCR
- Microscopy
- Rapid Diagnostic Tests (RDT)
By Product
- Malaria Diagnostic Kits
- Reagents
- Instruments
By End Use
- Hospitals & Clinics
- Diagnostic Centers
- Academic and Research Institutes
Regional Analysis
The Middle East and Africa region led the global malaria diagnostics market in 2023, capturing 53.10% of the total market share. This dominance is attributed to the region’s disproportionately high malaria burden, accounting for over 90% of global cases. Market growth is being driven by strengthened governmental initiatives and robust support from international bodies such as the World Health Organization (WHO) and the Global Fund, which emphasize early detection and effective treatment.
Advancements in diagnostic technologies, particularly Rapid Diagnostic Tests (RDTs) and molecular methods, are improving both reach and accuracy of testing. Enhanced healthcare infrastructure and increased funding have enabled broader implementation, especially in remote and underserved areas. Moreover, public-private partnerships are playing a key role in expanding access to diagnostic tools.
Rising public awareness regarding the importance of early diagnosis and timely treatment in endemic areas continues to support market demand. Ongoing research and innovation aimed at enhancing diagnostic sensitivity, specificity, and cost-effectiveness are expected to further strengthen the region’s malaria control strategies.
Emerging Trends
- Expansion of Rapid Diagnostic Test (RDT) Use: Rapid diagnostic tests have become the cornerstone of malaria diagnosis in low-resource settings. In 2020, WHO-prequalified manufacturers supplied approximately 419 million RDTs globally to national programmes, with the majority destined for sub-Saharan Africa. The ease of use and minimal infrastructure requirements have driven this expansion.
- Adoption of Molecular Point-of-Care Tests (LAMP): Loop-mediated isothermal amplification (LAMP) assays are being introduced as a simple molecular diagnostic that can detect low-density and sub-microscopic infections without complex equipment. LAMP can be performed at the point of care and has demonstrated high accuracy in field evaluations.
- Digitalization of RDT Interpretation: Smartphone-based readers and dedicated devices are increasingly used to reduce human error in RDT reading and to enable real-time data reporting. For example, HealthPulse, a smartphone mRDT reader app, has been deployed to guide health workers through test administration and interpretation. Dedicated hardware like the Deki Reader™ automates RDT analysis and uploads results to central databases, supporting quality control.
- Regulatory Milestones for Molecular Tests: Regulatory bodies are approving molecular screening tests for malaria. In March 2024, the U.S. Food and Drug Administration granted clearance for Roche’s Cobas Malaria molecular assay to screen donated blood for malaria parasites, marking the first such approval in the United States.
Use Cases
- Routine Clinical Diagnosis in Sub-Saharan Africa: In 2017, 276 million RDT units were sold by manufacturers, up from 48 million in 2008, reflecting widespread adoption. By 2017, an estimated 75 % of all malaria tests in sub-Saharan Africa were performed using RDTs, compared with 40 % in 2010.
- Community Health Worker Support via Mobile App: In Busia County, Kenya, a pilot of the HealthPulse app involved 200 public-sector Community Health Volunteers and 23 private clinic healthcare workers. The app provided step-by-step guidance and digitized test results, improving correct test administration and timely decision-making.
- Quality Control and Real-Time Reporting: In the Democratic Republic of the Congo (2022), Deki Reader devices were implemented across multiple sites. In one study, the reader achieved a concordance rate of 99.0 % (95 % CI 98.4–99.4) with expert visual interpretation and offered automated uploads of test results for immediate malaria surveillance.
- Elimination-Phase Surveillance with LAMP: In a field evaluation in Northwest Ethiopia, a non-instrumented LAMP assay (NINA-LAMP) was used to screen 150 consecutive febrile patients. The assay showed comparable performance to nested PCR, detecting sub-microscopic infections that would have been missed by microscopy alone.
Conclusion
The global malaria diagnostics market is poised for steady growth, underpinned by technological advancements, rising public health initiatives, and strong international collaboration. Rapid Diagnostic Tests (RDTs) continue to lead diagnostic efforts due to their simplicity and accessibility, particularly in sub-Saharan Africa.
The growing adoption of molecular techniques, digital tools, and mobile health innovations is enhancing diagnostic accuracy and real-time disease tracking. With strong support from government programs and global health organizations, and increasing emphasis on early detection, the market is expected to play a critical role in malaria control, surveillance, and eventual eradication efforts worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



