Table of Contents
Overview
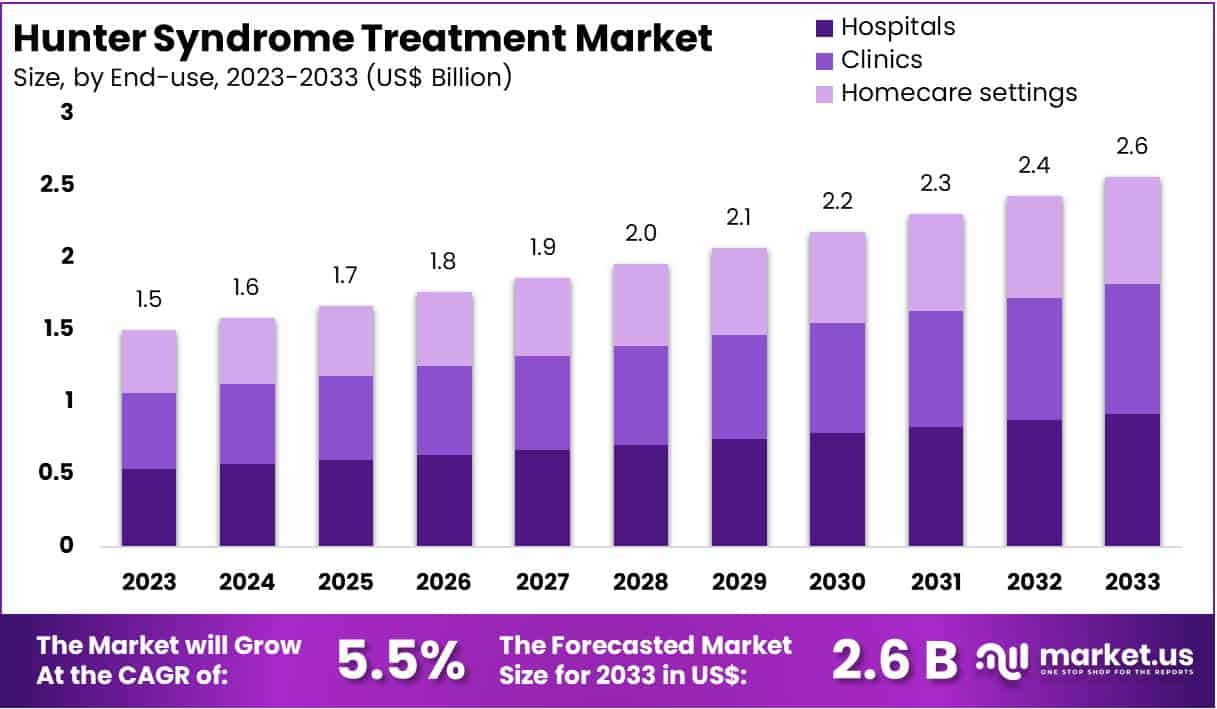
New York, NY – July 25, 2025 – The Global Hunter Syndrome Treatment Market size is expected to be worth around US$ 2.6 Billion by 2033, from US$ 1.5 Billion in 2023, growing at a CAGR of 5.5% during the forecast period from 2024 to 2033. North America maintained a leading position in the market, accounting for over 38% of the share, with a market value of approximately US$ 0.5 billion.
The global Hunter Syndrome Treatment Market is witnessing significant growth as the demand for advanced therapies increases due to the rising diagnosis of this rare genetic disorder. Hunter syndrome, also known as mucopolysaccharidosis type II (MPS II), is a severe condition that affects various organs and systems, causing progressive physical and cognitive decline. This rare disease primarily affects males and has a profound impact on patients’ quality of life.
Recent advancements in enzyme replacement therapy (ERT), gene therapy, and substrate reduction therapy (SRT) are expected to drive the market’s growth. These therapies aim to alleviate symptoms, improve patient outcomes, and potentially slow disease progression, offering new hope to those affected. ERT, particularly with approved drugs such as idursulfase, remains a cornerstone of treatment, though emerging therapies like gene therapy are showing promising potential to provide long-term benefits.
The Hunter Syndrome Treatment Market is also being fueled by increasing awareness and early diagnosis. Healthcare systems globally are improving diagnostic practices and encouraging earlier intervention, which is expected to drive demand for therapies.
With a compound annual growth rate (CAGR) of over 8.4%, the market is expected to expand significantly in the coming years, offering lucrative opportunities for pharmaceutical companies and healthcare providers. Major players are focusing on innovative solutions to address unmet clinical needs, positioning the market for continuous development.
Key Takeaways
- The global Hunter Syndrome Treatment Market is forecasted to reach approximately US$ 2.6 billion by 2033, up from US$ 1.5 billion in 2023, reflecting a compound annual growth rate (CAGR) of 5.5% from 2024 to 2033.
- In 2023, Enzyme Replacement Therapy (ERT) was the dominant treatment type, capturing over 76% of the market share.
- The Hospitals segment led the end-user category in 2023, accounting for more than 76% of the market share in Hunter Syndrome Treatment.
- North America held the largest market share in 2023, commanding over 38% of the global Hunter Syndrome Treatment Market.
Segmentation Analysis
- Treatment Type Analysis: In 2023, Enzyme Replacement Therapy (ERT) dominated the Hunter Syndrome Treatment Market, holding over 76% of the market share. ERT addresses enzyme deficiencies by administering synthetic enzymes, helping to manage the symptoms of Hunter Syndrome effectively. Its widespread use reflects its reliability and acceptance as the primary treatment. While Hematopoietic Stem Cell Transplant (HSCT) holds a smaller share, it plays a crucial role in providing potential cures, especially for severe cases or patients unresponsive to ERT.
- End-use Analysis: Hospitals led the Hunter Syndrome Treatment Market in 2023, with more than 76% of the market share. These facilities are essential for managing the complex disorder, offering comprehensive care for diagnosis, treatment, and ongoing management. Hospitals provide access to specialized healthcare professionals and advanced technologies, making them central to the treatment process. Clinics, while playing a role in diagnosis and initial treatment, have a smaller market share due to the intensive care required for Hunter Syndrome. Homecare settings, though growing, remain the smallest segment.
Market Segments
By Treatment Type
- Enzyme replacement therapy (ERT)
- Hematopoietic stem cell transplant (HSCT)
By End-use
- Hospitals
- Clinics
- Homecare settings
Regional Analysis
In 2023, North America held a dominant position in the Hunter Syndrome Treatment Market, accounting for over 38% of the market share, valued at US$ 0.5 billion. This leadership can be attributed to high rates of diagnosis and treatment, along with advanced healthcare infrastructure and increased awareness of genetic disorders. These factors promote early detection and intervention, driving demand for effective therapies.
The presence of major pharmaceutical companies in the region further supports market growth. These companies invest extensively in research and development, introducing innovative treatments tailored to the complexities of Hunter Syndrome. Their continuous efforts ensure a steady supply of advanced therapeutic options, reinforcing the market’s expansion.
Government initiatives also play a key role in the market’s growth. Financial support and favorable policies aimed at rare diseases enhance access to vital treatments. Subsidies and targeted health programs improve patient care, ensuring that necessary therapies reach those in need.
Collaborations between research institutions and healthcare providers strengthen the treatment landscape, accelerating the development and adoption of new therapies. These partnerships contribute to North America’s leadership in setting high standards for treatment efficacy and patient care in the Hunter Syndrome Treatment Market.
Emerging Trends
- One-time Gene Therapy Approaches: Recent clinical studies are evaluating AAV-based gene therapies that deliver a functional IDS gene in a single dose. For example, the RGX-121 trial in children aged five years and over is investigating whether one administration can restore enzyme activity and reduce treatment burden compared to weekly infusions.
- Intrathecal Enzyme Replacement to Address Brain Symptoms: Enzyme replacement delivered directly into the spinal fluid (intrathecal ERT) has been pursued to cross the blood–brain barrier. Idursulfase-IT received FDA orphan designation on September 3, 2009, for potential neurocognitive benefits, marking a shift toward treating central nervous system involvement.
- Substrate Reduction Therapy (SRT): Small molecules that inhibit glycosaminoglycan (GAG) synthesis are under investigation to reduce storage in cells. Early studies suggest SRT may lower substrate levels and complement enzyme therapies, offering an oral treatment option in the future.
- Hematopoietic Stem Cell and Gene-Edited Cell Therapies: Strategies combining hematopoietic stem cell transplantation with gene editing aim to give patients’ own blood cells the corrected gene. These approaches are designed to provide a continuous source of enzyme throughout the body, including hard-to-reach tissues.
- Fusion Protein and Transporter-Enhanced Therapies: Novel fusion proteins like Tividenofusp Alfa (DNL310) are being tested in pediatric participants. These constructs use transport domains to improve enzyme delivery into key cells, potentially enhancing clinical outcomes with fewer infusions.
Use Cases
- Weekly Idursulfase in Young Children: In a 53-week safety trial, children aged 16 months to 4 years (n=20) and 5 to 7.5 years (n=8) received weekly intravenous idursulfase at 0.5 mg/kg. The treatment was generally well tolerated; infusion reactions such as rash occurred in 7% of patients, and nausea in 5%.
- Improvement in Adults’ Walking and Lung Function: Adult patients treated with idursulfase showed an average increase of 54.5 meters in the six-minute walk test and a 3.8 percentage-point rise in forced vital capacity after regular infusions. Left ventricular mass index also decreased by 12.4%, indicating cardiac benefits.
- Idursulfase Beta vs. Placebo: In a 52-week study of 24 male Asian participants (mean age 12 years), idursulfase beta outperformed placebo: walking distance improved by 62.2 m versus 7.3 m, urinary GAG levels fell by 71.1% versus a 21.4% increase, and liver and spleen volumes decreased by about 26.7% and 26.5%, respectively.
Conclusion
The global Hunter Syndrome Treatment Market is on a growth trajectory, driven by advancements in enzyme replacement therapy, gene therapy, and substrate reduction therapy. With the market expected to reach US$ 2.6 billion by 2033, significant opportunities lie in innovative therapies and improved diagnostics.
North America’s market dominance, fueled by advanced healthcare infrastructure and regulatory support, positions it as a key player. Emerging trends such as gene therapies, intrathecal enzyme replacement, and gene-edited stem cell therapies offer promising solutions for improving patient outcomes. As research and treatment options evolve, the market is poised for continued expansion and enhanced patient care.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



