Table of Contents
Overview
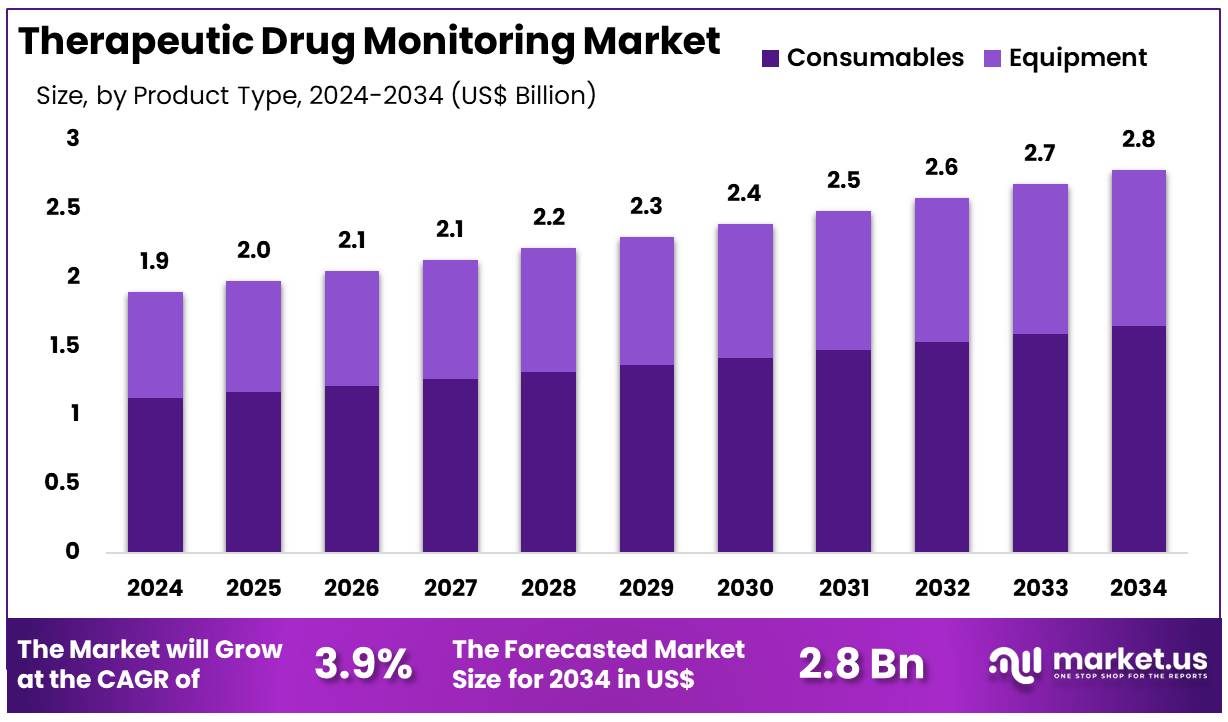
New York, NY – Dec 05, 2025 – Global Therapeutic Drug Monitoring Market size is expected to be worth around US$ 2.8 Billion by 2034 from US$ 1.9 Billion in 2024, growing at a CAGR of 3.9% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 41.4% share with a revenue of US$ 0.8 Billion.
Therapeutic Drug Monitoring (TDM) has been recognized as a critical clinical practice that supports the optimization of drug therapy and the enhancement of patient safety. The technique involves the measurement of specific drug concentrations in a patient’s bloodstream, enabling precise adjustment of dosage levels. The growth of TDM adoption has been attributed to the rising prevalence of chronic diseases, the expansion of personalized medicine, and the increasing use of narrow-therapeutic-index drugs.
The demand for TDM has been strengthened by its ability to improve treatment outcomes, minimize adverse drug reactions, and support evidence-based clinical decision-making. Its application is widely observed in the management of conditions such as epilepsy, cancer, cardiovascular disorders, and infectious diseases. Advancements in immunoassay technologies, chromatographic techniques, and point-of-care testing have further accelerated TDM utilization across healthcare settings.
The global TDM market has experienced steady expansion as healthcare providers prioritize safer and more effective therapeutic approaches. The integration of TDM into clinical workflows has been facilitated by technological innovation, increased laboratory automation, and growing emphasis on precision dosing. Continuous research and development activities are expected to contribute to broader accessibility and improved test efficiency.
Therapeutic Drug Monitoring continues to serve as an essential component of modern healthcare by supporting individualized treatment strategies and strengthening patient management practices worldwide.
Key Takeaways
- In 2024, the therapeutic drug monitoring market generated revenues of US$ 3.9 billion, registering a CAGR of 3.9%, and is projected to reach US$ 2.8 billion by 2034.
- By product type, the market is classified into consumables and equipment, with consumables accounting for the leading share of 59.3% in 2024.
- Based on technology, the market is segmented into immunoassays and chromatography-spectrometry, where immunoassays held a dominant 56.5% share.
- In terms of drug class, the market is divided into antiepileptic drugs, antiarrhythmic drugs, immunosuppressant drugs, antibiotic drugs, and others, with antiepileptic drugs emerging as the leading segment at 45.7% revenue share.
- Regarding end users, the market is categorized into hospitals, diagnostic laboratories, and research & academic institutes, with hospitals leading at 53.9% market share.
- North America dominated the global landscape, securing a 41.4% share in 2024.
Regional Analysis
North America has been observed as the leading region in the Therapeutic Drug Monitoring (TDM) market, accounting for a 41.4% revenue share. The dominance of the region can be attributed to the high prevalence of chronic diseases requiring precise medication management and the growing number of organ transplant procedures.
Autoimmune conditions affect a notable share of the US population, with more than 15 million individuals, or 4.6%, diagnosed with at least one autoimmune disease as of June 2022. Such widespread conditions increase the need for individualized dosing supported by TDM. In Canada, 3,369 solid organ transplants were reported in 2023, up from 2,886 in 2022, further strengthening the demand for TDM in post-transplant care. The continued revenue growth of major diagnostics companies also reflects rising adoption of testing solutions across the region.
Asia Pacific is projected to register the fastest CAGR during the forecast period, driven by an expanding elderly population and increased healthcare investments. The region’s demographic shift has resulted in a higher chronic disease burden, including 233.03 million diabetes patients in China in 2023. Rising healthcare expenditure in major markets such as India and the significant prevalence of epilepsy in Japan reinforce the growing need for advanced drug monitoring solutions across Asia Pacific.
Emerging Trends
- AI-Driven Precision Dosing: The integration of artificial intelligence and machine learning into therapeutic drug monitoring workflows is accelerating. These systems support model-informed precision dosing by processing large datasets including electronic health records and pharmacokinetic parameters to forecast individual drug exposure. As a result, optimized dosing recommendations are generated, improving safety and therapeutic effectiveness in clinical decision support environments.
- Pharmacometabolomics and Pharmacogenetics: Expanded use of pharmacometabolomic profiling is enabling the characterization of patient-specific metabolic signatures that influence drug behavior. When combined with pharmacogenetic assessment of metabolic gene variants, this approach is strengthening personalized therapy by identifying individuals predisposed to subtherapeutic exposure or heightened toxicity before treatment is initiated.
- Advanced Mass Spectrometry Platforms: Liquid chromatography tandem mass spectrometry is increasingly replacing conventional immunoassays in clinical laboratories. Its heightened sensitivity, specificity, and analytical throughput permit accurate drug quantification, including compounds without native chromophores. Workflow efficiencies are further improved through automated sample preparation and integrated online extraction systems.
Use Cases
- Baricitinib Monitoring in Pediatric Patients: A validated LC-MS/MS procedure quantifies baricitinib concentrations in plasma using a 50 µL sample across a range of 1.024–100 ng/mL. Reported mean levels of 11.25 ± 10.86 ng/mL have supported dose optimization in juvenile rheumatoid arthritis, ensuring adequate exposure while reducing toxicity risks.
- Simultaneous Measurement of Mycophenolic Acid and Triazoles: A multiplex LC-MS/MS assay has been applied to measure mycophenolic acid and triazole antifungal agents in serum. Its broad analytical range and low detection thresholds assist clinicians in adjusting therapy for transplant patients and individuals with invasive fungal infections to maintain concentrations within defined therapeutic limits.
- Rapid Colistin Therapeutic Drug Monitoring: A high-speed LC-MS/MS method for colistin uses a 1 mL plasma sample and completes analysis within four minutes. The rapid turnaround and analytical precision facilitate timely monitoring in critically ill patients, helping maintain concentrations within the narrow therapeutic index to balance antimicrobial efficacy and nephrotoxicity risk.
Frequently Asked Questions on Therapeutic Drug Monitoring
- What is Therapeutic Drug Monitoring?
Therapeutic Drug Monitoring is a clinical practice in which drug concentrations are measured in patient samples to optimize dosing. The approach ensures effective therapeutic exposure, reduces toxicity risks, and supports individualized treatment decisions across various high-risk medication categories. - Why is Therapeutic Drug Monitoring important?
TDM is considered essential because drug responses vary widely among patients. Its use enables dosage adjustments based on measured levels, enhancing treatment efficacy, preventing adverse reactions, and supporting precision medicine initiatives in healthcare systems with growing complex therapeutic regimens. - Which drugs commonly require Therapeutic Drug Monitoring?
TDM is mainly applied to drugs with narrow therapeutic windows such as antiepileptics, immunosuppressants, antibiotics, and cardiovascular agents. These drugs require close monitoring to maintain safe plasma concentrations that support therapeutic outcomes while minimizing potential toxicity. - What is driving growth in the Therapeutic Drug Monitoring market?
Market growth is driven by rising chronic disease prevalence, increasing adoption of precision medicine, and expanding use of high-risk drugs requiring monitoring. Advancements in analytical technologies further strengthen market demand across both developed and emerging healthcare systems. - Which regions dominate the Therapeutic Drug Monitoring market?
North America maintains dominance due to strong laboratory networks, advanced healthcare infrastructure, and high adoption of precision therapies. Europe follows closely, while Asia-Pacific shows rapid expansion supported by rising healthcare investment and growing incidence of drug-managed chronic diseases. - What technological trends are shaping the TDM market?
Technological progress includes widespread use of liquid chromatography–mass spectrometry, automation in sample processing, and development of point-of-care testing systems. These innovations improve accuracy, shorten turnaround times, and support broader implementation of individualized therapeutic monitoring strategies. - Who are the key players in the TDM market?
Leading companies include Abbott, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, and Bio-Rad Laboratories. Their portfolios include immunoassay systems, chromatography solutions, and laboratory automation tools that support comprehensive therapeutic drug monitoring applications.
Conclusion
The Therapeutic Drug Monitoring market continues to advance as healthcare systems prioritize individualized treatment and safer medication practices. Growth has been supported by rising chronic disease prevalence, increased use of narrow-therapeutic-index drugs, and expansion of precision medicine initiatives. Technological progress in immunoassays, mass spectrometry, and automated laboratory workflows has strengthened diagnostic capabilities and improved clinical decision-making.
North America maintains a strong lead, while Asia Pacific exhibits rapid expansion driven by demographic shifts and higher healthcare investment. Overall, TDM is expected to remain integral to optimizing therapeutic outcomes, reducing adverse events, and enhancing patient management across global healthcare settings.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



