Table of Contents
Overview
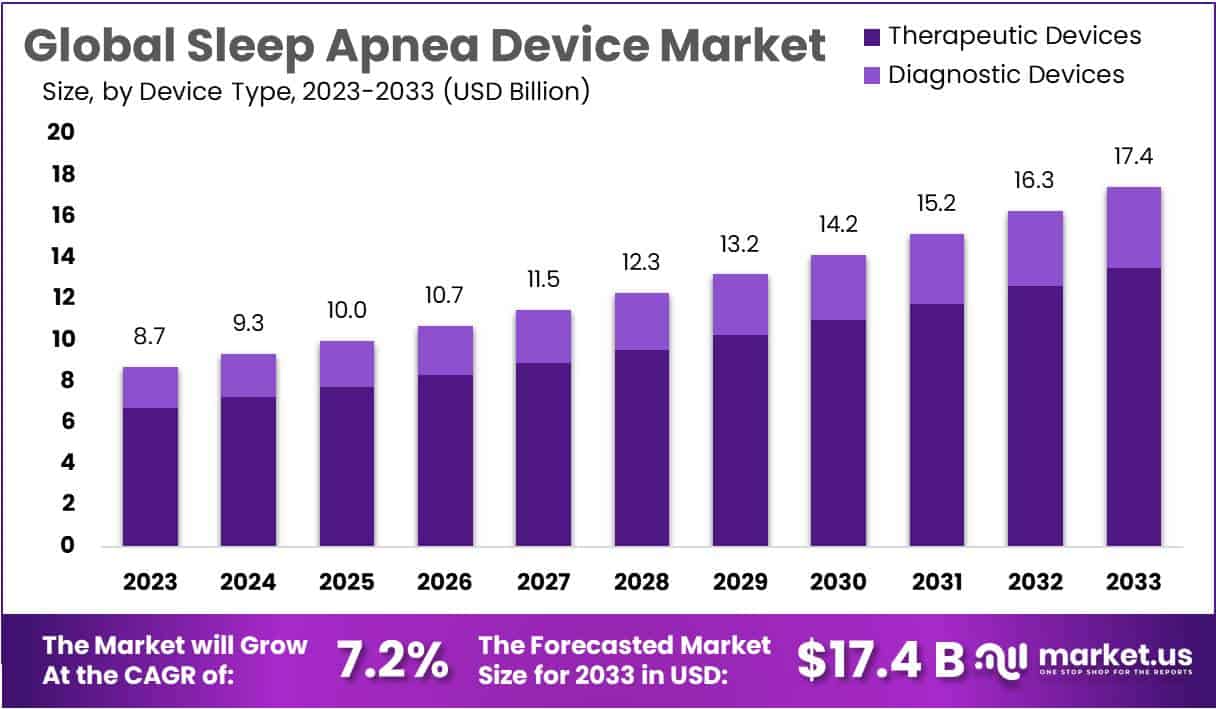
New York, NY – Jan 12, 2026 – The Global Sleep Apnea Devices Market size is expected to be worth around USD 17.4 Billion by 2033, from USD 8.7 Billion in 2023, growing at a CAGR of 7.2% during the forecast period from 2024 to 2033.
Sleep apnea devices are medical technologies designed to support effective breathing during sleep for individuals diagnosed with sleep apnea, a condition characterized by repeated interruptions in breathing. The adoption of these devices has increased steadily, driven by rising awareness of sleep-related disorders and their long-term health implications.
The most commonly used sleep apnea devices include Continuous Positive Airway Pressure (CPAP) devices, Bi-level Positive Airway Pressure (BiPAP) devices, Auto-adjusting Positive Airway Pressure (APAP) devices, oral appliances, and adaptive servo-ventilation systems. CPAP devices remain the standard treatment option, delivering a constant flow of air to keep the airway open during sleep. Oral appliances are increasingly adopted among patients with mild to moderate conditions due to their portability and ease of use.
The growth of the sleep apnea devices segment can be attributed to increasing prevalence of obesity, aging populations, and improved diagnostic rates. Technological advancements, such as quieter motors, compact designs, and digital connectivity for therapy monitoring, have further enhanced patient compliance and treatment outcomes. Devices integrated with cloud-based data tracking are enabling healthcare providers to monitor patient adherence more effectively.
From a market perspective, demand is supported by expanding home healthcare settings and favorable reimbursement policies in developed economies. Emerging markets are also witnessing gradual growth due to improving healthcare infrastructure and rising awareness levels.
Overall, sleep apnea devices are positioned as essential therapeutic tools within respiratory care, with continued innovation expected to support sustained market expansion over the coming years.
Key Takeaways
- In 2023, the global sleep apnea devices market was valued at USD 8.7 billion.
- The market size is projected to reach USD 17.4 billion by 2033.
- The sleep apnea devices market is expected to expand at a CAGR of 7.2% during the forecast period from 2024 to 2033.
- In 2019, approximately 170 million individuals in the United States were diagnosed with obstructive sleep apnea.
- Nearly 40% of the global obese population is affected by sleep apnea.
- Around 70% of individuals diagnosed with sleep apnea are classified as obese.
- The average cost of sleep apnea devices, such as CPAP and PSG systems, in the U.S. ranges between USD 600 and USD 800.
- Therapeutic devices account for the largest share of the global sleep apnea devices market, contributing 76.8% of total revenue.
- North America represents the leading regional market, holding a revenue share of 47.6% globally.
- According to the American Academy of Sleep Medicine, sleep apnea affects approximately 2–5% of women and 3–7% of men worldwide.
- The diagnostic devices segment includes respiratory polygraphs, polysomnography (PSG) systems, actigraphs, and pulse oximeters.
Sleep Apnea Devices Statistics
- Prevalence of Sleep Apnea: Sleep apnea impacts nearly 22 million individuals in the United States, with an estimated 80% of moderate to severe cases remaining undiagnosed, highlighting a substantial gap in screening and diagnosis.
- CPAP Usage: Continuous Positive Airway Pressure therapy remains the standard treatment approach, with more than 5 million CPAP devices prescribed annually, reflecting its strong clinical acceptance and effectiveness in managing obstructive sleep apnea.
- Mobile Health Applications: The adoption of smartphone-based health applications has increased significantly, with clinical studies indicating up to a 30% improvement in patient adherence to positive airway pressure therapy through digital engagement tools.
- Home Sleep Testing: Home-based sleep apnea testing has gained traction due to cost efficiency, with average prices ranging from $150 to $500, compared to approximately $1,000 for traditional in-laboratory polysomnography testing.
- Biological Aging Impact: Clinical evidence suggests that untreated sleep apnea accelerates biological aging processes, while consistent CPAP therapy has been shown to slow or partially reverse age-related physiological deterioration.
- Hypoglossal Nerve Stimulation: Hypoglossal nerve stimulation has emerged as a viable alternative for CPAP-intolerant patients, demonstrating a 20% improvement in treatment success rates among appropriately selected patient populations.
- Mandibular Advancement Devices: Mandibular advancement devices have proven effective in treating mild to moderate sleep apnea, delivering approximately a 50% reduction in apnea-related breathing events during sleep.
- Telemedicine Integration: Telehealth adoption for sleep apnea diagnosis and management increased by nearly 40% during the COVID-19 pandemic, accelerating remote consultations, therapy monitoring, and patient follow-up services.
- Smart Technology Integration: Sleep apnea devices equipped with smart features, including auto-adjusting pressure and remote monitoring, have contributed to a 25% increase in patient satisfaction and therapy compliance.
- Wearable Devices: The use of wearable sleep monitoring devices has expanded by approximately 35% in recent years, driven by growing consumer interest in continuous sleep tracking and early disorder detection.
- Surgical Interventions: Advances in minimally invasive surgical techniques have led to a 15% increase in their adoption for treating severe sleep apnea, particularly in patients unresponsive to conventional therapies.
- Comorbidity Management: Integrated care approaches addressing sleep apnea alongside comorbid conditions such as hypertension and diabetes have improved overall patient outcomes by nearly 20%.
- Insurance Coverage: Insurance coverage for sleep apnea treatments has broadened, with approximately 90% of health insurance plans now covering essential therapies, including PAP devices and diagnostic testing.
Regional Analysis
In 2023, North America led the global sleep apnea devices market, accounting for a dominant revenue share of 49.2% and generating a market value of USD 4.28 billion. This strong regional performance can be attributed to the presence of a well-established healthcare infrastructure. Countries such as the United States and Canada host a large number of key players in the sleep apnea devices market, supported by advanced healthcare systems and high adoption of medical technologies.
According to the American Academy of Sleep Medicine, sleep apnea affects approximately 2–5% of women and 3–7% of men worldwide. Rising awareness regarding sleep apnea has resulted in increased diagnosis rates, which in turn is driving demand for sleep apnea devices across North America. In addition, higher disposable incomes and the widespread use of respiratory care devices are contributing significantly to regional market growth.
Following North America, the Asia Pacific region is expected to witness substantial growth at a notable CAGR over the forecast period. Factors such as a rapidly expanding geriatric population and a rising incidence of respiratory and breathing-related disorders are supporting the growth of the sleep apnea devices market in the Asia Pacific region.
Use Cases
- Treatment of Obstructive Sleep Apnea: Continuous Positive Airway Pressure devices are widely recognized as the primary therapy for obstructive sleep apnea. By delivering a constant airflow through a mask, these devices prevent airway collapse, improve sleep quality, reduce daytime fatigue, and lower long-term health risks.
- Nerve Stimulation Devices: Implantable nerve stimulation devices provide an alternative for patients who are unable to tolerate CPAP therapy. These systems stimulate the hypoglossal nerve to maintain airway patency during sleep and are particularly effective in moderate to severe obstructive sleep apnea cases.
- Home Sleep Testing: Portable diagnostic devices enable sleep apnea evaluation within a home setting, improving patient convenience and diagnostic access. These systems monitor airflow, oxygen saturation, and respiratory patterns, supporting accurate diagnosis while reducing dependence on costly in-laboratory sleep studies.
- Veterans’ Health Applications: Sleep apnea devices are extensively utilized within veterans’ healthcare programs to address sleep-related disorders caused by physical strain and psychological stress. Their use improves sleep quality, supports long-term health management, and enhances overall quality of life for veterans.
- Addressing Comorbid Conditions: Effective sleep apnea management contributes to improved outcomes in associated conditions such as hypertension, diabetes, and cardiovascular disease. By stabilizing breathing during sleep, these devices support better blood pressure control, glucose regulation, and cardiovascular health.
- Remote Monitoring and Connectivity: Advanced sleep apnea devices with wireless connectivity allow real-time therapy monitoring and data sharing. This enables healthcare providers to track treatment adherence, adjust settings remotely, and intervene promptly, improving therapy effectiveness and patient engagement.
- Management of Central Sleep Apnea: Central sleep apnea is treated using advanced devices such as adaptive servo-ventilation systems, which dynamically adjust airflow based on breathing patterns. This approach improves treatment precision and safety for patients with neurologically driven breathing irregularities.
- Enhancing Patient Compliance: Modern device innovations focus on improving comfort and usability to increase long-term adherence. Features such as auto-adjusting pressure, quiet operation, and ergonomic mask designs reduce discomfort and encourage consistent therapy usage.
- Support for Pregnant Women: Sleep apnea devices are used to manage sleep-disordered breathing during pregnancy, helping reduce risks such as gestational diabetes and preeclampsia. Effective treatment supports maternal health while promoting improved fetal outcomes and safer deliveries.
Frequently Asked Questions on Sleep Apnea Devices
- What are sleep apnea devices and how do they work?
Sleep apnea devices are medical products designed to maintain open airways during sleep by delivering pressurized air or repositioning the jaw or tongue, thereby reducing breathing interruptions and improving oxygen flow throughout the night. - What are the main types of sleep apnea devices available?
The primary types of sleep apnea devices include continuous positive airway pressure devices, bilevel positive airway pressure devices, automatic positive airway pressure devices, oral appliances, and adaptive servo-ventilation systems, each targeting specific patient severity and comfort requirements. - Who should use sleep apnea devices?
Sleep apnea devices are recommended for patients diagnosed with obstructive, central, or complex sleep apnea, particularly individuals experiencing loud snoring, daytime fatigue, cardiovascular risks, or oxygen desaturation during sleep as confirmed by clinical sleep studies. - Are sleep apnea devices effective for long-term treatment?
Sleep apnea devices are considered effective for long-term management when used consistently, as they significantly reduce apnea-hypopnea index levels, improve sleep quality, lower cardiovascular risks, and enhance overall patient quality of life. - What factors influence the selection of a sleep apnea device?
The selection of a sleep apnea device is influenced by apnea severity, patient anatomy, comfort preferences, lifestyle factors, portability needs, noise levels, and physician recommendations based on diagnostic sleep study outcomes. - What is driving growth in the sleep apnea devices market?
Growth in the sleep apnea devices market is driven by rising prevalence of sleep disorders, increasing obesity rates, aging populations, improved diagnostic awareness, and technological advancements in portable and patient-friendly respiratory therapy solutions. - Which device segment dominates the sleep apnea devices market?
The continuous positive airway pressure device segment dominates the sleep apnea devices market due to its widespread clinical acceptance, high efficacy in treating obstructive sleep apnea, and strong reimbursement coverage across developed healthcare systems. - How is technological innovation shaping the market?
Technological innovation is shaping the market through smart connectivity, cloud-based compliance monitoring, quieter motors, improved mask designs, and miniaturized travel-friendly devices, enhancing patient adherence and enabling remote clinical management. - Which regions show strong demand for sleep apnea devices?
North America shows strong demand due to advanced healthcare infrastructure and high diagnosis rates, while Asia-Pacific is experiencing rapid growth supported by expanding healthcare access, rising awareness, and increasing disposable income levels.
Conclusion
The sleep apnea devices market represents a critical and expanding segment within global respiratory care, driven by rising disease prevalence, obesity rates, and aging populations. Continuous advancements in therapeutic and diagnostic technologies have improved treatment effectiveness, patient compliance, and remote monitoring capabilities.
Strong demand from home healthcare settings, favorable reimbursement frameworks in developed regions, and increasing awareness in emerging economies continue to support market growth. With CPAP devices maintaining clinical dominance and innovation accelerating across smart and connected solutions, sleep apnea devices are expected to remain essential tools in managing sleep-disordered breathing and associated comorbidities over the long term.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



