Table of Contents
Overview
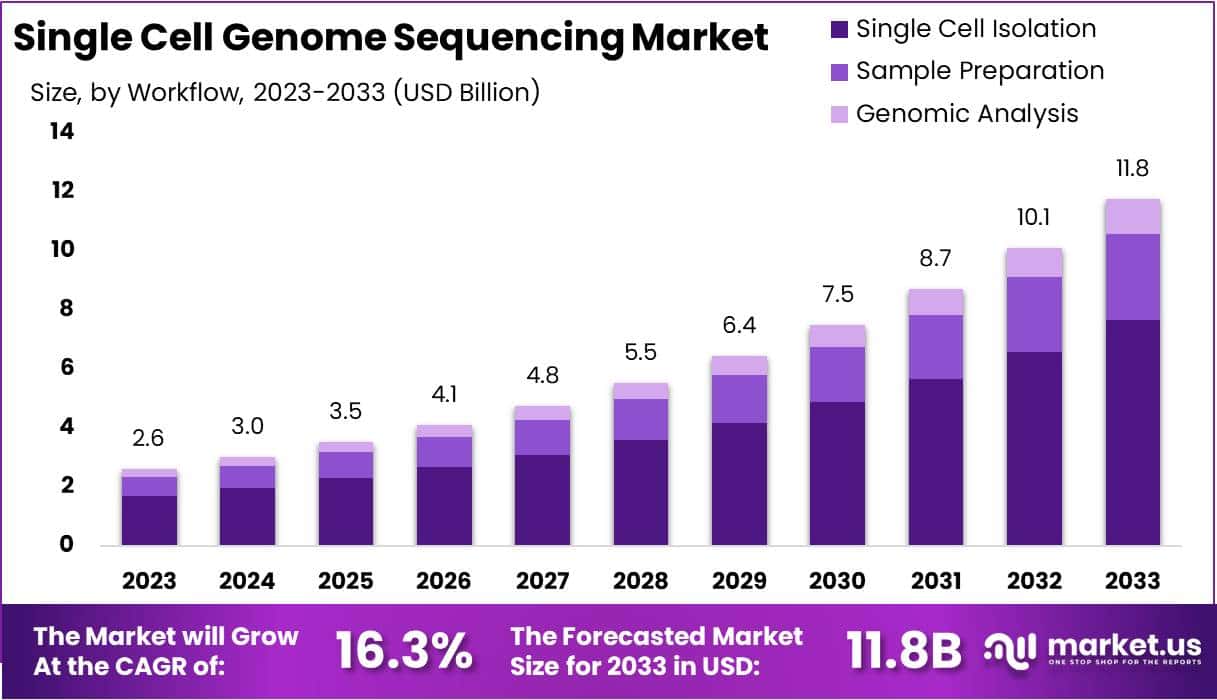
New York, NY – Feb 04, 2026 – The Global Single cell Genome Sequencing Market size is expected to be worth around USD 11.8 Billion by 2033, from USD 2.6 Billion in 2023, growing at a CAGR of 16.3% during the forecast period from 2024 to 2033.
Single-cell genome sequencing represents a major advancement in modern genomics, enabling the detailed analysis of genetic information at the resolution of individual cells. Unlike traditional bulk sequencing methods, which analyze large populations of cells together, single-cell genome sequencing allows each cell’s DNA to be examined independently. This approach provides a clearer understanding of cellular diversity, genetic variation, and functional differences within complex biological systems.
The basic workflow of single-cell genome sequencing begins with the isolation of individual cells from a tissue, blood sample, or microbial population. This step is typically achieved using microfluidics, fluorescence-activated cell sorting, or manual cell picking. Once isolated, the genetic material within each cell is extracted. Because a single cell contains a very small amount of DNA, whole genome amplification is performed to generate sufficient material for sequencing while maintaining genomic integrity.
Following amplification, sequencing libraries are prepared and processed using high-throughput sequencing platforms. The resulting data are then analyzed using advanced bioinformatics tools to reconstruct genomes, identify mutations, and assess genomic heterogeneity across cells.
Single-cell genome sequencing has become a critical tool across multiple research areas, including oncology, developmental biology, immunology, and microbiology. It supports the identification of rare cell populations, the study of tumor evolution, and the understanding of disease mechanisms at unprecedented resolution.
As adoption increases, single-cell genome sequencing is expected to play an essential role in precision medicine, biomarker discovery, and next-generation diagnostics, supporting more accurate and targeted healthcare solutions.
Key Takeaways
- Market Growth: The market is projected to expand from USD 2.6 billion in 2023 to approximately USD 11.8 billion by 2033, registering a compound annual growth rate (CAGR) of 16.3% over the forecast period.
- Technology Dominance: PCR-based technologies accounted for around 29% of total market revenue in 2023, supported by their high accuracy and effectiveness in amplifying limited DNA quantities in single-cell applications.
- Instrument Segment: Instruments represented nearly 58% of the market share in 2023, driven by continuous technological improvements in sequencing systems, amplification platforms, and cell encapsulation devices.
- Genomic Analysis: The genomic analysis workflow segment held more than 65% of the market share in 2023, reflecting its critical importance in identifying and interpreting genetic variations at the single-cell level.
- Cancer Research: Cancer-related applications led the market in 2023 with approximately 35% share, as single-cell sequencing is widely used to analyze tumor heterogeneity and support the development of targeted therapies.
- End-User Segment: Academic and research institutions accounted for over 68% of market revenue in 2023, primarily due to extensive use of single-cell sequencing in studies of cellular diversity and complexity.
- North America: North America dominated the market in 2023 with a 47% share, valued at around USD 1.2 billion, supported by strong investments in advanced genomic research infrastructure.
Key Statistics and Advances
- DNA Content and Amplification Performance
- Human diploid cells contain approximately 6 picograms (pg) of genomic DNA.
- SMOOTH-seq technology enables robust whole-genome amplification using as little as 10 pg of input DNA.
- Post-amplification DNA fragments generated through SMOOTH-seq extend up to 6 kilobases (kb) following purification.
- Circular consensus sequencing (CCS) outputs typically exceed 1 kb, with maximum read lengths reaching 43,693 base pairs (bp).
- The average polymerase read length is approximately 60 kb, reflecting high sequencing fidelity.
- CCS reads demonstrate a 96% mapping rate to the human reference genome.
- Genome coverage achieved by CCS reads ranges from 10.6% to 41.3%, with a mean coverage of 19.5% per cell.
- Copy Number Variation and Structural Variant Detection
- Copy number variation (CNV) profiles derived from K562 single cells are highly concordant with bulk sequencing data.
- Structural variant (SV) detection using SMOOTH-seq achieves 75.2%–76.9% precision.
- Insertion variants show the highest detection accuracy, with an average precision of 84%.
- Polymerase chain reaction (PCR) validation confirms approximately 90% of selected extrachromosomal circular DNA (ecDNA) candidates.
- The largest deletion event identified spans 89,252 bp, supported by sequencing data from eight independent cells.
- Advances in Single-Cell Whole Genome Sequencing (scWGS)
- Recent scWGS methodologies enable accurate analysis of DNA fragments up to 20 kb in length.
- The digital multiple displacement amplification (dMDA) reaction significantly reduces amplification bias, enhancing data consistency.
- Five single-cell samples processed via dMDA yielded 1–4 micrograms (µg) of amplified DNA per cell.
- PacBio HiFi sequencing produces up to 20 gigabases (Gb) of data per single cell, with average read lengths between 2.8 and 3.6 kb.
- Coverage uniformity achieved with dMDA is substantially higher than that observed with conventional multiple displacement amplification (MDA).
- Microsatellite Profiling and Mutation Analysis
- Early single-cell studies evaluated fewer than 100 microsatellite loci per cell, whereas recent approaches enable profiling of up to 12,000 loci.
- Microsatellite mutations function as lineage-specific markers, uniquely labeling each cell division.
- Aneuploidy rates in human sperm range from 1% to 5%, with elevated mis-segregation occurring during the second meiotic division.
- Single-cell DNA sequencing of human oocytes reveals aneuploidy rates between 18% and 70%, primarily driven by reverse chromatid segregation.
- Integration of Genomics and Epigenomics Technologies
- Single-cell Hi-C methods detect over 1 million chromatin contacts per cell, enabling high-resolution analysis of genome compartmentalization.
- ATAC-seq, utilizing Tn5 transposase, supports high-throughput chromatin accessibility profiling across thousands to hundreds of thousands of cells.
- Tn5-based workflows also facilitate DNA methylation analysis and enable CNV detection at megabase-scale resolution.
- GpC methyltransferase–based techniques deliver fine-scale chromatin state mapping, identifying DNA methylation patterns at 25 bp intervals.
- Technologies such as LIANTI and META-CS improve single-nucleotide variant (SNV) detection accuracy by sequencing both DNA strands for reciprocal error correction.
- SHARE-seq and third-generation sequencing (TGS) approaches provide integrated insights into single-cell epigenomes and transcriptomes, with TGS capturing long-range epigenetic modifications across chromatin states.
Biotechnology and Healthcare Market Outlook
- Sequencing and Genomics Markets
- The global next-generation sequencing (NGS) market is projected to grow from USD 8 billion in 2022 to USD 29 billion by 2032, registering a CAGR of 14.6%.
- The DNA sequencing market is expected to expand from USD 10.1 billion in 2023 to USD 40.5 billion by 2033, at a 15.3% CAGR.
- The sequencing reagents market is forecasted to increase from USD 6.9 billion in 2023 to USD 14.4 billion by 2033, growing at a 7.6% CAGR.
- The protein sequencing market is anticipated to rise from USD 3.2 billion in 2023 to USD 4.7 billion by 2033, with a 3.7% CAGR.
- Cell Therapy and Related Markets
- The CAR-T cell therapy market is expected to grow from USD 2.8 billion in 2023 to USD 10.2 billion by 2033, reflecting a 13.8% CAGR.
- The autologous cell therapy market, valued at USD 5.5 billion in 2022, is projected to reach USD 33.1 billion by 2032, with a 20.2% CAGR.
- The cell-based assays market is forecasted to expand from USD 16.3 billion in 2022 to USD 37.1 billion by 2032, growing at 8.8% CAGR.
- The stem cell therapy market is expected to increase from USD 11.1 billion in 2022 to USD 44.5 billion by 2032, achieving a 15.3% CAGR.
- The live cell imaging market is projected to grow from USD 2.8 billion in 2022 to USD 6.7 billion by 2032, at a 9.4% CAGR.
- The cell dissociation market is anticipated to rise from USD 382.7 million in 2023 to USD 1,141.2 million by 2032, registering a 13.3% CAGR.
- The cell and gene therapy market is expected to expand from USD 10.7 billion in 2022 to USD 78 billion by 2032, reflecting a strong 22.6% CAGR.
- The sickle cell disease treatment market is projected to grow from USD 1.9 billion in 2022 to USD 6.9 billion by 2032, with a 14.1% growth rate.
Regional Analysis
In 2023, North America held a leading position in the single-cell genome sequencing market, accounting for more than 47% of global revenue and reaching a market value of approximately USD 1.2 billion. This leadership is primarily attributed to strong investments in genomic research, well-established healthcare infrastructure, and the widespread adoption of next-generation sequencing (NGS) technologies.
Furthermore, consistent funding from both government bodies and private institutions, along with the strong presence of major industry participants, has reinforced the region’s market leadership. Continuous advancements in genomic research and innovation remain key drivers supporting market expansion in North America.
Europe represents the second-largest regional market, supported by a rapidly expanding biotechnology sector and a growing number of research programs. Favorable government policies encouraging genomic research, combined with increasing emphasis on personalized medicine, are contributing to market growth. Key countries such as Germany, the United Kingdom, and France play a significant role in advancing genomic analysis and technological development across the region.
The Asia-Pacific market is expected to register the fastest growth rate over the forecast period. This growth is driven by rising research investments, improving healthcare infrastructure, and increasing awareness of genomic technologies. Countries including China, Japan, and India are emerging as major contributors, supported by expanding research capabilities and growing adoption of advanced sequencing solutions.
Use Cases
- Disease Diagnosis and Management: Single-cell genome sequencing improves disease diagnosis by identifying cell-specific genetic variants linked to cancer and rare disorders. This precision enables personalized treatment strategies, enhances therapeutic effectiveness, and reduces adverse effects. Accurate targeting of pathogenic mutations supports improved clinical outcomes in complex and heterogeneous disease conditions.
- Research on Cellular Diversity: Single-cell sequencing enables comprehensive analysis of cellular heterogeneity across tissues and biological systems. It supports understanding of cell-type–specific functions, interactions, and regulatory pathways. These insights are essential for advancing research in autoimmune, infectious, and inflammatory diseases, and for identifying novel therapeutic targets.
- Drug Development and Testing: Single-cell genome sequencing provides high-resolution insights into cellular responses to drug candidates. It supports evaluation of treatment efficacy and toxicity at the cellular level, particularly in oncology. This approach enables development of targeted therapies, improves drug success rates, and reduces variability in pharmacological outcomes.
- Advancements in Genomic Research: Single-cell genome sequencing advances genomic research by delivering detailed insights into individual cellular genomes. It strengthens understanding of genetic architecture, regulation, and variation. This capability supports innovations in genetic engineering, accelerates discovery, and underpins future applications of genomics in medicine and biotechnology.
Frequently Asked Questions on Single cell Genome Sequencing
- How does single cell genome sequencing differ from bulk sequencing?
Unlike bulk sequencing, which averages genetic information across millions of cells, single cell genome sequencing captures cell-specific genomic variations, allowing precise detection of mutations, copy number variations, and clonal diversity at single-cell resolution. - What are the major applications of single cell genome sequencing?
Single cell genome sequencing is widely applied in cancer research, developmental biology, neuroscience, and immunology, where it supports tumor heterogeneity analysis, lineage tracing, somatic mutation detection, and understanding of complex cellular ecosystems. - What technologies are used in single cell genome sequencing?
Key technologies include whole genome amplification methods, next-generation sequencing platforms, microfluidics, and droplet-based systems, which collectively enable efficient isolation, amplification, and high-throughput sequencing of individual cellular genomes. - What is the current scope of the single cell genome sequencing market?
The single cell genome sequencing market encompasses instruments, reagents, consumables, and bioinformatics solutions, supporting research and clinical applications across oncology, precision medicine, and life science research institutions worldwide. - What factors are driving growth in the single cell genome sequencing market?
Market growth is driven by rising cancer incidence, increasing adoption of precision medicine, technological advancements in sequencing platforms, and growing research investments in genomics and cell-based analysis across academic and pharmaceutical sectors. - Who are the primary end users in the market?
Primary end users include academic research institutes, biotechnology and pharmaceutical companies, clinical research organizations, and diagnostic laboratories that utilize single cell sequencing to support drug discovery, biomarker identification, and translational research. - How is technological innovation influencing the market?
Continuous innovation in microfluidics, automation, and high-throughput sequencing has improved scalability, reduced costs, and enhanced data quality, thereby expanding accessibility and accelerating adoption of single cell genome sequencing solutions globally.
Conclusion
Single-cell genome sequencing has emerged as a transformative technology in modern genomics by enabling high-resolution analysis of genetic variation at the individual cell level. Its ability to reveal cellular heterogeneity, rare mutations, and disease mechanisms has made it indispensable across cancer research, developmental biology, and precision medicine.
Strong market growth is being driven by continuous technological innovation, expanding research applications, and rising investment in genomics. As sequencing accuracy improves and costs gradually decline, single-cell genome sequencing is expected to play a central role in next-generation diagnostics, targeted therapies, and advanced biomedical research worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



