Table of Contents
Overview
New York, NY – Feb 13, 2026 – The Global Point of Care Glucose Testing Market size is expected to be worth around US$ 6.3 Billion by 2033, from US$ 3.9 Billion in 2023, growing at a CAGR of 4.9% during the forecast period from 2024 to 2033.
The global point-of-care (POC) glucose testing market is witnessing consistent expansion, supported by the increasing prevalence of diabetes and the growing demand for rapid diagnostic solutions. Point-of-care glucose testing enables immediate blood glucose measurement at or near the site of patient care, facilitating timely clinical decisions and improved patient outcomes.
According to industry estimates, the rising incidence of type 1 and type 2 diabetes has significantly increased the adoption of portable glucose monitoring devices across hospitals, clinics, and homecare settings. The market growth can be attributed to technological advancements in biosensors, enhanced device accuracy, and user-friendly digital interfaces. Additionally, the integration of wireless connectivity and data management systems has strengthened remote patient monitoring capabilities.
Healthcare systems are increasingly emphasizing early diagnosis and routine monitoring to reduce diabetes-related complications. As a result, the demand for compact, cost-effective, and minimally invasive glucose testing solutions has accelerated. Emerging economies are also contributing to market expansion due to improved healthcare infrastructure and rising awareness regarding diabetes management.
Leading manufacturers continue to invest in product innovation and regulatory approvals to strengthen their competitive positioning. With supportive reimbursement frameworks and ongoing research initiatives, the point-of-care glucose testing market is expected to maintain stable growth over the forecast period, reinforcing its critical role in modern diabetes care management.
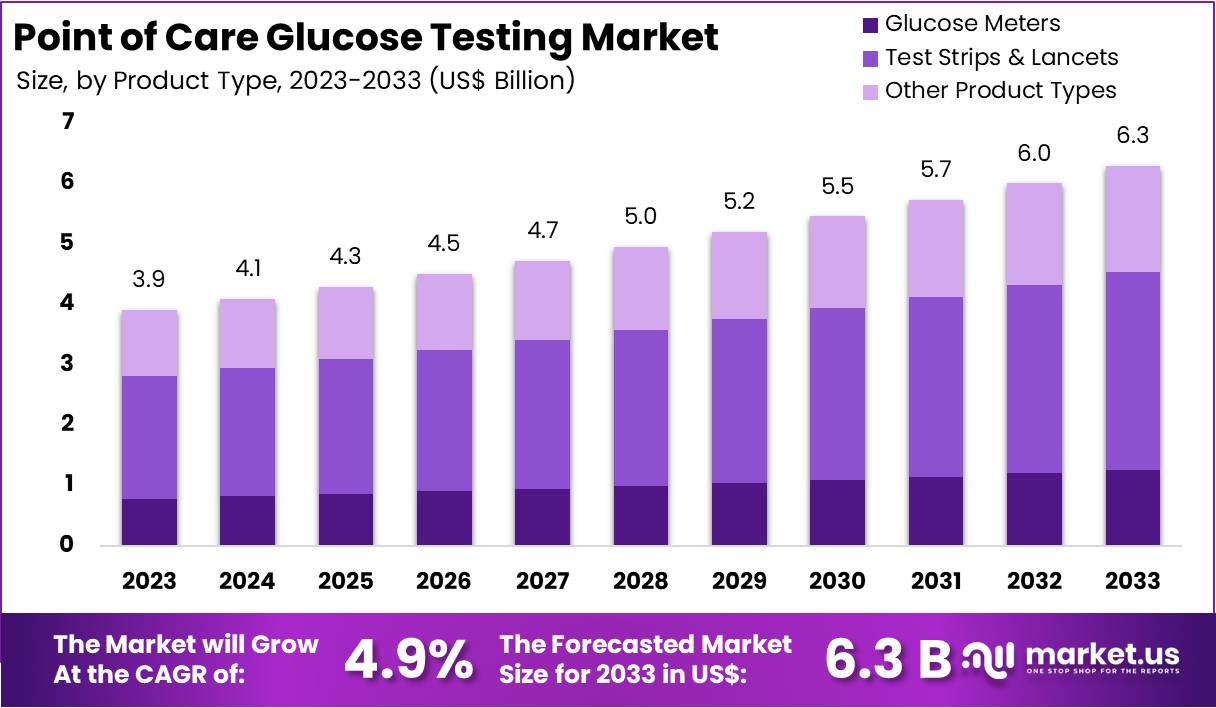
Key Takeaways
- The Point of Care Glucose Testing market is anticipated to expand from US$ 3.9 billion in 2023 to approximately US$ 6.3 billion by 2033, reflecting a compound annual growth rate (CAGR) of 4.9% over the forecast period.
- In 2023, Test Strips & Lancets emerged as the leading product category, contributing more than 52.1% of the overall market revenue.
- The Type-2 Diabetes segment represented the largest application area in 2023, accounting for a dominant 63.5% share of the total market.
- North America held the leading regional position in 2023, capturing 41.4% of the global market share, with a recorded market value of US$ 0.4 billion.
Regional Analysis
In 2023, North America secured a leading position in the point of care glucose testing market, accounting for over 41.4% of the global share and generating approximately US$ 0.4 billion in revenue. Market dominance has been supported by the high prevalence of diabetes across the region, which has created sustained demand for reliable and rapid glucose monitoring solutions. The presence of well-established healthcare infrastructure has facilitated the swift adoption and integration of advanced diagnostic technologies.
Strong financial support from both public and private healthcare sectors has further contributed to market expansion. Continuous investments in medical innovation have accelerated product development and improved accessibility to point of care testing devices. Rising awareness regarding diabetes prevention and management has encouraged proactive health monitoring among patients.
In addition, supportive regulatory frameworks have strengthened product reliability and user confidence. Ongoing technological advancements, particularly digital integration in medical devices, are expected to sustain regional leadership over the forecast period.
Emerging Trends
- Over the counter continuous monitoring for non insulin users: In 2023, the U.S. Food and Drug Administration cleared the first integrated continuous glucose monitoring system designed for adults not using insulin. This approval expanded direct consumer access, enabling individuals aged 18 and older to monitor glucose levels continuously without prescription requirements.
- Expanded quality and safety guidelines in clinical settings: Updated infection prevention guidelines have strengthened safety protocols for point of care glucose testing devices in healthcare facilities. Emphasis has been placed on single use, auto disabling fingerstick devices to minimize cross contamination risks in hospitals and long term care environments.
- Enhanced quality management practices: In 2024, institutional reviews emphasized the importance of structured quality management systems in point of care glucose testing programs. Standardized procedures before, during, and after testing were recommended to improve analytical accuracy, ensure regulatory compliance, and enhance overall patient safety outcomes.
- Broader use beyond hospital wards: Point of care glucose testing has expanded into non acute environments, including long term care facilities and community health programs. Simplified device operation and structured staff training frameworks have supported broader adoption beyond centralized laboratories and traditional inpatient hospital wards.
Use Cases
- Inpatient glycemic management: Among hospitalized patients with diabetes or treatment induced hyperglycemia, point of care glucose testing is commonly performed every four to six hours. Frequent monitoring supports rapid therapeutic adjustments, reducing risks associated with uncontrolled blood sugar fluctuations in acute clinical settings.
- Home self monitoring: Home based glucose monitoring remains a critical component of diabetes management strategies. Regular testing enables individuals to identify glucose patterns and adjust dietary or lifestyle habits accordingly. In the United States, 52 percent of adults reported undergoing glucose testing within three years.
- Continuous monitoring without prescription: The over the counter continuous glucose monitoring system authorized in 2023 is utilized by adults aged 18 and above managing diabetes without insulin therapy. Continuous real time glucose readings generated at frequent intervals support proactive lifestyle modifications and therapeutic optimization.
- Point of care in long term care facilities: In long term care facilities, adoption of single use, auto disabling glucose testing devices has aligned with infection control guidance from public health authorities. On site testing conducted by trained nursing personnel enhances operational efficiency while reducing contamination risks.
Frequently Asked Questions on Point of Care Glucose Testing
- How does Point of Care Glucose Testing work?
Point of Care Glucose Testing operates through small handheld glucometers that analyze capillary blood obtained via fingerstick. The device uses enzymatic biosensors to detect glucose concentration and provides digital readings within seconds for timely clinical intervention. - What are the key advantages of Point of Care Glucose Testing?
The primary advantages include rapid results, improved patient monitoring, enhanced workflow efficiency, and reduced laboratory dependency. Immediate glucose readings support faster treatment adjustments, particularly in emergency departments, intensive care units, and outpatient settings. - Where is Point of Care Glucose Testing commonly used?
Point of Care Glucose Testing is widely utilized in hospitals, ambulatory surgical centers, physician offices, and home care environments. It is especially critical for managing diabetes, monitoring critically ill patients, and assessing glucose fluctuations during surgical procedures. - What factors influence the accuracy of Point of Care Glucose Testing?
Accuracy is influenced by hematocrit levels, temperature variations, improper sampling, device calibration, and interfering substances. Regular quality control measures and adherence to clinical protocols are essential to maintain consistent and reliable glucose readings. - What is driving growth in the Point of Care Glucose Testing Market?
Market growth is driven by the increasing prevalence of diabetes, rising geriatric population, demand for rapid diagnostics, and expansion of home healthcare services. Technological advancements in biosensors and digital connectivity further contribute to sustained market expansion. - Who are the major end users in the Point of Care Glucose Testing Market?
Hospitals represent the dominant end-user segment due to high patient volume and acute care requirements. However, home healthcare and self-monitoring segments are expanding rapidly, driven by patient preference for convenient and cost-effective glucose monitoring solutions. - What technological trends are shaping the Point of Care Glucose Testing Market?
Key trends include integration with digital health platforms, wireless data transmission, advanced enzymatic sensors, and improved strip accuracy. Continuous product innovation is enhancing user convenience, reducing errors, and enabling real-time data sharing with healthcare providers.
Conclusion
The global point of care glucose testing market is positioned for steady expansion, supported by the growing burden of diabetes and rising demand for rapid, decentralized diagnostic solutions. Technological advancements in biosensors, digital integration, and continuous monitoring systems have enhanced device accuracy and patient convenience.
Strong regional performance, particularly in North America, reflects advanced healthcare infrastructure and supportive regulatory frameworks. Expanding applications across hospitals, homecare, and long term care settings further reinforce market penetration. With ongoing product innovation, quality standardization, and increasing awareness of proactive diabetes management, sustained growth is expected throughout the forecast period.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



