Table of Contents
Overview
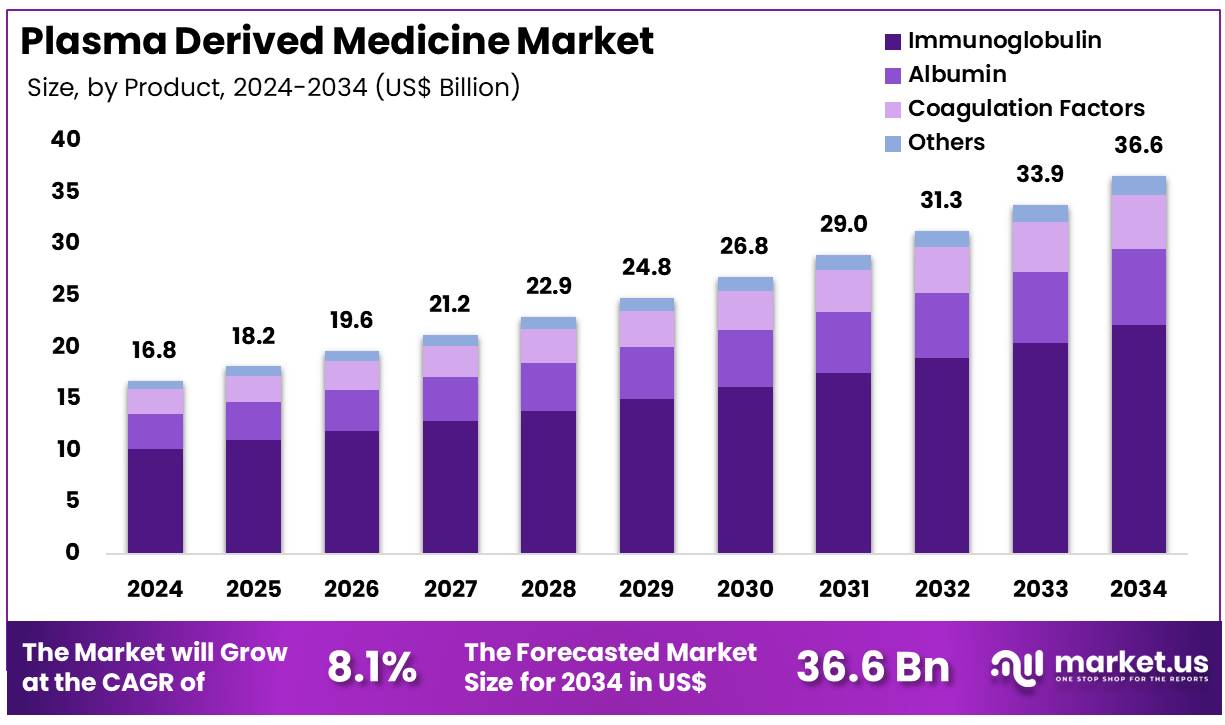
New York, NY – Dec 11, 2025 – Global Plasma Derived Medicine Market size is expected to be worth around US$ 36.6 Billion by 2034 from US$ 16.8 Billion in 2024, growing at a CAGR of 8.1% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 37.2% share with a revenue of US$ 6.2 Billion.
The development of plasma-derived medicines has been recognized as a significant advancement in modern healthcare. These therapies are produced through the careful fractionation of human plasma, enabling the extraction of essential proteins used in the treatment of various life-threatening conditions. The growing adoption of these products has been driven by their established efficacy in managing immunodeficiency disorders, bleeding conditions, and rare genetic diseases.
The demand for plasma-derived therapies has been supported by the expanding prevalence of chronic and immune-related disorders. Rising diagnostic rates and improved healthcare access have contributed to increased utilization across global markets. The growth of the sector has further been influenced by continuous enhancements in plasma collection, purification technologies, and compliance with stringent safety standards, ensuring high-quality medicinal outputs.
Significant attention has been directed toward immunoglobulins, coagulation factors, and albumin, as these products account for the largest share of plasma-based therapeutics. Their use has been associated with improved patient outcomes, particularly in cases where alternative treatments are limited or unavailable. The expansion of plasma-collection infrastructure and the advancement of bioprocessing capabilities are expected to strengthen supply stability and support long-term market development.
Continued investment in plasma-derived medicine is anticipated to play a pivotal role in addressing critical unmet medical needs worldwide. As the industry evolves, emphasis on innovation, donor safety, and regulatory compliance will remain central to ensuring sustained therapeutic value for patients around the globe.
Key Takeaways
- The market for plasma-derived medicine generated revenue of US$ 16.8 billion in 2024, supported by a CAGR of 8.1%, and is projected to reach US$ 36.6 billion by 2034.
- The product landscape is segmented into immunoglobulin, albumin, coagulation factors, and others, with immunoglobulin accounting for 60.5% of the market share in 2024.
- In terms of application, the market is categorized into bleeding disorders, multifocal motor neuropathy, liver disease, and others, with bleeding disorders representing 63.5% of the total share.
- North America*dominated the regional landscape, achieving a 37.2% share in 2024.
Regional Analysis
North America accounted for the largest revenue share of 37.2%, supported by rising diagnosis rates of rare and chronic diseases that require plasma-derived therapies and by the steady recovery of plasma collection volumes. Conditions such as primary immunodeficiencies, hemophilia, and neurological disorders increasingly depend on these treatments. The Immune Deficiency Foundation notes that about 250,000 individuals in the United States live with primary immunodeficiency, many of whom require immunoglobulin therapy. Expanded efforts by plasma collection centers in the United States and Canada have strengthened supply availability to meet growing patient needs.
Industry performance further reflects this momentum. Grifols reported Biopharma revenue of EUR 1,521 million in the first quarter of 2025, rising 9.6% year over year at constant currency, driven by a 17.5% increase in immunoglobulin revenue. CSL Behring recorded a 5% rise in revenue to US$8.483 billion for the half-year ending December 31, 2024, supported by strong demand for immunoglobulins used in conditions such as primary immune deficiency and CIDP.
Asia Pacific is anticipated to record the fastest CAGR, supported by improved healthcare infrastructure, increasing diagnostic capabilities, and stronger governmental focus on rare diseases. Regional initiatives in China and Japan, together with growing investment from leading producers such as Takeda, are expected to accelerate adoption of plasma-derived medicines across the region.
Use Cases
- Treatment of Immune Deficiency Disorders: Plasma-derived immunoglobulins are used to support patients with weak or deficient immune systems. These therapies strengthen immune response, reduce infection risk, and improve overall health. Their use has become essential for managing primary and secondary immune deficiencies effectively.
- Hemophilia and Bleeding Disorders: Plasma-derived clotting factors enable patients with hemophilia to control bleeding episodes safely. These factors help prevent complications, support surgical procedures, and reduce life-threatening risks. Their use has improved patient stability, daily functioning, and long-term health outcomes.
- Autoimmune Disease Management: Plasma-derived products regulate abnormal immune activity in autoimmune disorders. These therapies reduce inflammation, alleviate symptoms, and improve neuromuscular function in conditions like Guillain-Barré syndrome. Their use supports overall disease management and enhances patient quality of life.
- Support in Critical Care and Surgery: Albumin, a key plasma protein, maintains blood volume during surgery and critical care. It stabilizes circulation, supports organ function, and reduces complications linked to fluid loss. Its clinical use improves recovery outcomes in trauma, emergencies, and major operations.
- Neurological Conditions: Plasma therapies help manage immune-related neurological disorders by reducing inflammation affecting nerves. Patients with conditions such as CIDP experience improved strength, reduced pain, and better mobility. These products play a vital role in stabilizing neurological function and enhancing life quality.
Frequently Asked Questions on Plasma Derived Medicine
- How are plasma-derived medicines produced?
Production involves collecting donated plasma, conducting pathogen screening, and separating plasma proteins through fractionation and purification steps. These steps ensure high product safety, consistent quality, and compliance with strict regulatory standards established by global health authorities. - Which conditions are treated with plasma-derived medicines?
Plasma-derived medicines are used for primary immune deficiencies, hemophilia, von Willebrand disease, autoimmune disorders, and acute trauma cases. Their therapeutic value has been recognized due to strong clinical evidence supporting improved patient outcomes in rare and chronic medical conditions. - How safe are plasma-derived medicines?
Safety is assured through donor screening, pathogen testing, viral inactivation, and adherence to stringent manufacturing protocols. These processes significantly reduce contamination risks, enabling regulated distribution across global markets with robust oversight from regulatory agencies. - Why is plasma donation important for these medicines?
Plasma donation is essential because plasma proteins cannot be manufactured synthetically. Consistent donor participation supports stable supply, enabling treatment continuity for patients dependent on these medicines for managing chronic, life-long medical conditions. - What is driving growth in the plasma-derived medicine market?
Market growth is driven by rising prevalence of immune disorders, expanding diagnostic coverage, improved healthcare infrastructure, and increased plasma collection capacity. These factors collectively support higher treatment adoption and sustained long-term demand across multiple geographic regions. - Which product categories dominate the market?
Immunoglobulins represent the leading product category due to strong clinical demand for immune deficiency treatments. Coagulation factors and albumin also maintain significant shares, supported by broad therapeutic application and consistent patient utilization globally. - Which regions show the highest market demand?
North America holds the largest demand due to advanced healthcare systems, high diagnostic rates, and extensive plasma collection networks. Europe and Asia-Pacific markets are expanding steadily, supported by improving access and rising investments in plasma-derived therapies.
Conclusion
The plasma-derived medicine market is positioned for sustained expansion, supported by rising chronic and immune-related disorders, strengthened diagnostic capabilities, and advancements in plasma collection and processing technologies. Demand for immunoglobulins, coagulation factors, and albumin continues to dominate due to their critical therapeutic roles in managing rare, life-threatening conditions.
Strong regional performance, particularly in North America and the rapidly growing Asia Pacific, reinforces market momentum. Ongoing investments in innovation, regulatory compliance, and donor safety are expected to ensure supply stability and enhance global treatment accessibility, supporting the long-term growth outlook of plasma-derived therapies.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



