Table of Contents
Overview
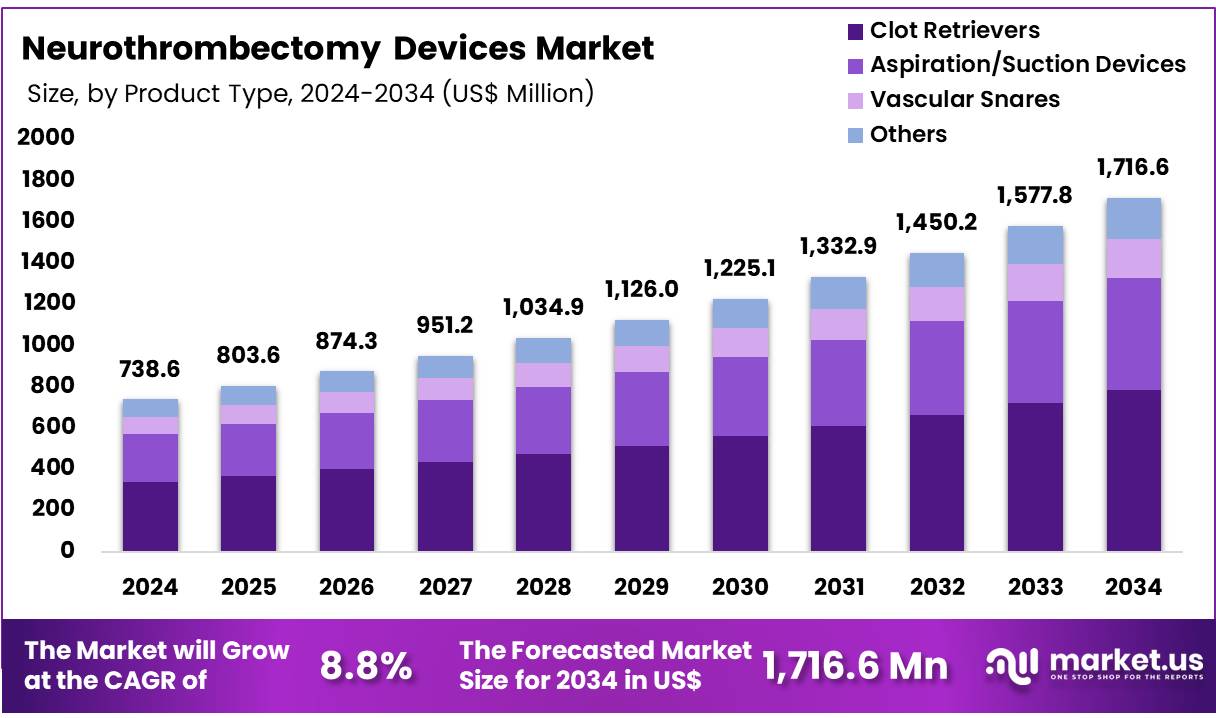
New York, NY – Dec 19, 2025 – Global Neurothrombectomy Devices Market size is expected to be worth around US$ 1,716.6 Million by 2034 from US$ 738.6 Million in 2024, growing at a CAGR of 8.8% during the forecast period 2025 to 2034. In 2024, Europe led the market, achieving over 32.7% share with a revenue of US$ 241.5 Million.
The neurothrombectomy devices market is formed around advanced medical technologies designed for the mechanical removal of blood clots from cerebral arteries. These devices are primarily used in the treatment of acute ischemic stroke, where rapid restoration of blood flow is critical to reduce neurological damage and mortality. The market formation has been driven by the rising global incidence of stroke, increasing aging population, and growing awareness of early stroke intervention.
Neurothrombectomy devices typically include stent retrievers, aspiration catheters, and ancillary access devices. Among these, stent retrievers have been widely adopted due to their proven clinical efficacy and strong outcomes in large-vessel occlusions. Continuous improvements in device design, such as enhanced flexibility, trackability, and clot integration, have contributed to broader clinical acceptance.
The market structure is shaped by strong participation from established medical device manufacturers, supported by robust research and development investments. Regulatory approvals from authorities such as the U.S. FDA and European CE marking have played a key role in accelerating commercialization and standardizing product quality. In parallel, favorable reimbursement frameworks in developed healthcare systems have supported hospital adoption.
From a regional perspective, North America has been positioned as a leading market due to advanced healthcare infrastructure and high procedure volumes. However, emerging markets in Asia-Pacific are witnessing steady growth, supported by improving stroke care facilities and expanding access to minimally invasive neurointerventional procedures.
Overall, the neurothrombectomy devices market formation reflects a convergence of clinical need, technological innovation, and supportive healthcare policies, establishing a strong foundation for sustained market expansion in the coming years.
Key Takeaways
- In 2024, the market for Neurothrombectomy Devices generated a revenue of US$ 738.6 million, with a CAGR of 8.8%, and is expected to reach US$ 1,716.6 million by the year 2034.
- The product type segment is divided into Clot Retrievers, Aspiration/Suction Devices, Vascular Snares, and Others with Clot Retrievers taking the lead in 2024 with a market share of 45.7%.
- By End-User, the market is bifurcated into Hospitals, Ambulatory Surgical Centers (ASCs), Emergency Clinics, and Specialty Clinics, with Hospitals leading the market with 62.7% of market share.
- Europe led the market by securing a market share of 32.7% in 2024.
Regional Analysis
The high prevalence of stroke, particularly acute ischemic stroke (AIS), across Europe most notably in Germany has been identified as a key driver of market growth. The region is supported by advanced healthcare infrastructure and a strong adoption rate of innovative medical technologies, which has facilitated the broad utilization of neurothrombectomy devices. As a result, Europe accounted for the largest share of the global market in 2024, capturing 32.7% of total revenue and maintaining its dominant position.
Major participants operating in the European neurothrombectomy devices market include Medtronic, Stryker Corporation, Penumbra Inc., and Acandis GmbH. These companies are actively pursuing product innovation, forming strategic collaborations, and expanding their regional footprint to address the rising demand for neurovascular intervention procedures.
In February 2024, CERENOVUS, Inc., a subsidiary of Johnson & Johnson MedTech, announced the launch of the CEREGLIDE™ 71 Intermediate Catheter. This next-generation catheter incorporates TruCourse™ technology and is designed to support revascularization procedures in patients with acute ischemic stroke.
As the latest addition to the CEREGLIDE Family of Catheters within the CERENOVUS STROKE SOLUTIONS™ portfolio, the CEREGLIDE 71 is optimized for efficient direct aspiration and the delivery of compatible stent retrievers, including the EMBOTRAP™ III Revascularization Device, into the neurovasculature. This advancement is expected to improve procedural efficiency and precision in stroke treatment.
Emerging Trends
- AI-Embedded Endovascular Systems: The integration of artificial intelligence into endovascular devices is gaining momentum, particularly for clot detection and procedural planning. AI-driven image analysis is being increasingly adopted to enhance diagnostic accuracy and optimize intervention strategies. In parallel, AI-integrated retrieval systems that combine machine-learning algorithms with advanced device mechanics are emerging as a leading technology category.
- Autonomous and Robotic Navigation: Reinforcement learning–based approaches are being developed to enable safe and autonomous manipulation of micro-catheters and micro-guidewires within cerebral vasculature. Simulation studies have demonstrated success rates of up to 96%, with procedure times reduced to as low as seven seconds while maintaining safe force thresholds. In addition, kinematic, contact-aware motion-planning frameworks allow robotic systems to autonomously navigate telescopic, pre-bent tools using pre-operative 3D imaging data, achieving consistent success in reaching target vessels such as the left common carotid artery.
- Miniaturization Enabled by Magnetic Micro-Catheters: Advances in miniaturization have led to the development of ultraflexible, inflatable magnetic micro-catheters that leverage blood flow dynamics and magnetic steering for navigation. Preclinical studies in porcine models have shown successful access to highly tortuous distal arteries with diameters as small as 180 µm, enabling superselective delivery of contrast agents or embolic materials.
- Ergonomic Improvements and Material Innovation: Manufacturers are increasingly emphasizing ergonomic design, enhanced flexibility, and the use of advanced materials to improve clot retrieval efficiency. These innovations support minimally invasive procedures, contributing to shorter recovery times and a lower risk of procedural complications.
- Enhanced Visualization and Imaging Capabilities: Next-generation endovascular devices are being developed with improved visualization and imaging features. These advancements support more precise device placement and clot retrieval, improving procedural control during interventions.
Key Use Cases
- Automated Stroke Interventions: AI and reinforcement learning technologies form the foundation for partially or fully automated endovascular interventions. Such systems have the potential to significantly reduce procedure duration and clinician radiation exposure while maintaining high safety standards.
- Navigation in Complex Vascular Anatomy: Magnetic micro-catheters enable access to extremely small or highly tortuous vessels that were previously considered inaccessible. This capability supports targeted treatment of distal artery hemorrhages and tumor-feeding vessels.
- Precision Drug and Gene Delivery: Micro-catheter platforms allow highly selective delivery of therapeutic agents directly to fine vascular territories. This approach is applicable to central nervous system disorders and may be extended to other organ systems.
- Improved Outcomes Through Ergonomics and Imaging: Enhanced device ergonomics and advanced imaging capabilities improve procedural accuracy and clot removal efficiency. These factors collectively contribute to reduced complication rates and improved patient outcomes.
Frequently Asked Questions on Neurothrombectomy Devices
- How do neurothrombectomy devices work?
Neurothrombectomy devices function by physically engaging, capturing, or aspirating thrombus material from blocked intracranial vessels. Techniques commonly include stent retrievers and aspiration systems, which restore blood flow with minimal vessel trauma. - What types of neurothrombectomy devices are available?
The main types include stent retrievers, aspiration catheters, and combined systems. Stent retrievers dominate usage due to high recanalization rates, while aspiration devices are increasingly adopted for their procedural simplicity and reduced treatment time. - What conditions are treated using neurothrombectomy devices?
These devices are primarily used to treat acute ischemic stroke caused by large vessel occlusion. Their application is supported by clinical guidelines for patients presenting within defined therapeutic time windows and appropriate imaging criteria. - What are the benefits of neurothrombectomy procedures?
Neurothrombectomy procedures are associated with improved functional outcomes, reduced mortality, and lower long-term disability rates. Early intervention significantly enhances recovery prospects, lowering healthcare burden related to post-stroke rehabilitation and chronic care. - What is the neurothrombectomy devices market?
The neurothrombectomy devices market comprises manufacturers, suppliers, and healthcare providers involved in the development and use of mechanical clot removal systems for stroke management, driven by rising stroke incidence and technological advancements. - What factors are driving market growth?
Market growth is driven by increasing prevalence of ischemic stroke, aging populations, improved awareness of endovascular therapies, and favorable clinical evidence supporting mechanical thrombectomy as a standard of care for large vessel occlusion. - Which regions dominate the neurothrombectomy devices market?
North America dominates the market due to advanced healthcare infrastructure, high adoption of minimally invasive procedures, and strong reimbursement frameworks. Europe follows closely, while Asia-Pacific is witnessing accelerated growth from expanding stroke care access. - What is the future outlook of the neurothrombectomy devices market?
The market outlook remains cautiously optimistic, supported by ongoing device innovation, expanding clinical indications, and improved stroke networks. Emerging economies are expected to contribute significantly as healthcare investment and awareness of advanced stroke treatments increase.
Conclusion
The neurothrombectomy devices market is firmly established around critical clinical demand, continuous technological innovation, and supportive healthcare systems. Rising incidence of acute ischemic stroke, combined with aging populations, has reinforced the importance of rapid mechanical clot removal.
Strong clinical evidence, regulatory approvals, and favorable reimbursement have accelerated adoption, particularly in developed regions. Ongoing advancements in device design, imaging, robotics, and AI integration are enhancing procedural efficiency and patient outcomes. With expanding access in emerging economies and sustained investment by leading manufacturers, the market is positioned for stable, long-term growth through 2034.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



