Table of Contents
Overview
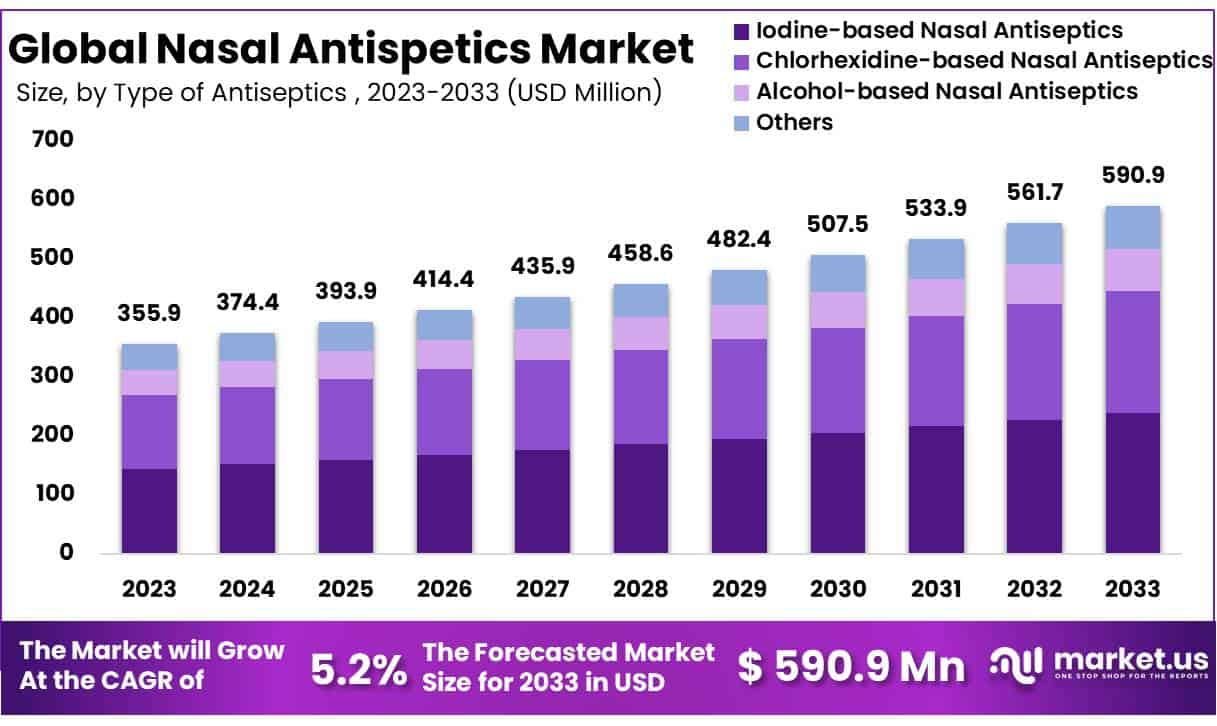
New York, NY – Feb 02, 2026 – The Global Nasal Antiseptics Market size is expected to be worth around USD 590.9 Million by 2033 from USD 355.9 Million in 2023, growing at a CAGR of 5.2% during the forecast period from 2024 to 2033.
Nasal antiseptics are topical formulations designed to reduce or eliminate pathogenic microorganisms present in the nasal cavity. These products are increasingly recognized as an effective preventive and adjunctive measure in infection control, particularly in healthcare and community settings. The nasal passage is known to act as a reservoir for bacteria and viruses, and targeted decolonization has been shown to support broader hygiene and disease prevention strategies.
The formulation of nasal antiseptics is typically based on well-established antimicrobial agents combined with biocompatible excipients. Common active ingredients include povidone-iodine, alcohol-based compounds, chlorhexidine derivatives, and other broad-spectrum antiseptic agents. These components are selected for their proven efficacy against bacteria, viruses, and fungi, while maintaining a favorable safety profile for repeated intranasal use.
Supporting ingredients such as purified water, buffering agents, humectants, and stabilizers are incorporated to ensure isotonicity, maintain pH balance, and enhance mucosal tolerance. The formulation is generally developed as a spray, gel, or swab-based solution to allow uniform distribution across the nasal mucosa and to support ease of administration.
From a regulatory and manufacturing perspective, nasal antiseptics are produced under stringent quality and safety standards. Clinical evaluations are conducted to assess antimicrobial effectiveness, mucosal safety, and user compliance. Growing awareness of infection prevention, coupled with rising demand for non-systemic antimicrobial solutions, is supporting the adoption of nasal antiseptics across hospitals, outpatient settings, and home care environments.
Overall, nasal antiseptics represent a scientifically grounded and practical approach to reducing microbial load and supporting public health initiatives focused on infection control.
Key Takeaways
- Market Size: The nasal antiseptics market is projected to reach approximately USD 590.9 million by 2033, increasing from USD 355.9 million in 2023.
- Market Growth: The market is anticipated to expand at a compound annual growth rate (CAGR) of 5.2% over the forecast period from 2024 to 2033.
- Type of Antiseptic Analysis: Iodine-based solutions continue to lead the nasal antiseptics market, accounting for nearly 40% of the overall market share.
- Formulation Analysis: Spray-based formulations held a prominent position in the global nasal antiseptics market, representing 42.1% of total revenue in 2023.
- Distribution Channel Analysis: Retail pharmacies remain the dominant distribution channel, capturing approximately 54.1% of the nasal antiseptics market share.
- Regional Analysis: North America is expected to demonstrate strong market performance, contributing an estimated 39.7% share of the global nasal antiseptics market by 2024.
Regional Analysis
The North American nasal antiseptics market is projected to experience notable growth, accounting for approximately 39.7% of the global market share by 2024. This expansion is primarily driven by the rising incidence of sinus-related infections, increasing public awareness through preventive healthcare campaigns, and sustained growth in healthcare expenditure across the region. In the United States alone, nearly 31 million individuals are affected by sinusitis annually, resulting in billions of dollars spent each year on over-the-counter products aimed at symptom management.
Market growth is further supported by continuous research and development efforts undertaken by key industry participants to introduce nasal antiseptic solutions that are both clinically effective and cost-efficient. These initiatives are strengthening product adoption across healthcare and consumer settings.
Additionally, ongoing urbanization, higher mobility due to increased travel, and densely populated living environments are contributing to the accelerated transmission of infections. As a result, nasal antiseptics are increasingly being adopted as a preventive measure to reduce microbial exposure and support infection control, particularly within the United States market.
Emerging Trends
- Growing Adoption of Alcohol-Based Nasal Antiseptics: Recent clinical evidence indicates an increasing shift toward alcohol-based nasal antiseptics as effective alternatives to conventional agents such as mupirocin and iodophors. Meta-analytical findings have demonstrated that these formulations are associated with a statistically significant reduction in surgical site infections (SSIs), supporting their expanding clinical acceptance.
- Inclusion in Comprehensive Infection Prevention Bundles: Nasal antiseptics are increasingly being integrated into multi-component infection prevention frameworks within healthcare settings. Structured approaches, such as the “7S Bundle,” have incorporated nasal antiseptics as a practical and effective measure for achieving nasal decolonization of both gram-positive and gram-negative organisms among patients and healthcare personnel.
- Application in the Prevention of Viral Respiratory Infections: The COVID-19 pandemic has accelerated research interest in nasal antiseptics for lowering viral burden in the upper respiratory tract. Ongoing clinical trials are assessing the role of antiseptic nasal sprays and gargles in reducing transmission risk of respiratory viruses, including SARS-CoV-2, particularly in high-exposure healthcare environments.
- Assessment of Povidone-Iodine for Nasal Decolonization: Povidone-iodine continues to be evaluated as a potential substitute for mupirocin in nasal decolonization protocols. However, available evidence indicates that nasal iodophor antiseptics did not achieve noninferiority compared with mupirocin in preventing Staphylococcus aureus clinical cultures among intensive care unit patients, limiting their current clinical positioning.
Key Use Cases
- Prevention of Surgical Site Infections: Nasal decolonization strategies using antiseptics, particularly mupirocin, have been shown to significantly reduce the incidence of SSIs, especially in high-risk procedures such as orthopedic and cardiac surgeries. Mupirocin remains the most extensively studied agent, with consistent evidence supporting its effectiveness in reducing S. aureus colonization in intensive care settings.
- Reduction of Healthcare-Associated Infections (HAIs): Universal decolonization protocols incorporating nasal antiseptics have been widely adopted to mitigate HAIs caused by methicillin-resistant Staphylococcus aureus (MRSA). Proactive treatment of colonized patients has been associated with a lower risk of progression to severe and life-threatening infections.
- Deployment in High-Risk and Congregate Settings: In environments characterized by close contact and elevated transmission risk, such as military and other congregate facilities, alcohol-based nasal antiseptics have been utilized as an evidence-based intervention. Their use has contributed to measurable reductions in infection rates among personnel, supporting broader application in similar high-density settings.
Frequently Asked Questions on Nasal Antiseptics
- How do nasal antiseptics work?
Nasal antiseptics work by disrupting microbial cell membranes or inactivating essential proteins of bacteria and viruses. This mechanism helps reduce nasal colonization, particularly of pathogens such as Staphylococcus aureus , thereby lowering infection transmission risk. - What are the common ingredients used in nasal antiseptics?
Common ingredients in nasal antiseptics include povidone-iodine, alcohol-based compounds, chlorhexidine derivatives, and essential antimicrobial agents. These ingredients are selected for broad-spectrum efficacy, safety for mucosal use, and rapid antimicrobial action. - Are nasal antiseptics safe for regular use?
Nasal antiseptics are generally safe when used as directed for short-term or procedural applications. However, prolonged or excessive use may cause mucosal irritation, dryness, or imbalance of normal nasal flora, requiring medical supervision. - What are the main applications of nasal antiseptics?
Nasal antiseptics are primarily used in pre-surgical decolonization, infection prevention in hospitals, outbreak control, and respiratory hygiene. Their use has expanded due to increased focus on reducing healthcare-associated infections and viral transmission. - What factors are driving the growth of the nasal antiseptics market?
The growth of the nasal antiseptics market can be attributed to rising hospital-acquired infections, increasing surgical procedures, and heightened awareness of infection prevention. The global emphasis on hygiene following pandemics has further supported market expansion. - Which end-users contribute most to market demand?
Hospitals and surgical centers account for the largest share of market demand due to routine preoperative use. Additional demand is generated by long-term care facilities, diagnostic centers, and home healthcare settings focused on infection prevention. - What future trends are expected in the nasal antiseptics market?
Future market trends include the development of gentler formulations, expansion into consumer healthcare, and increased research on antiviral efficacy. Strategic partnerships and regulatory approvals are expected to support innovation and long-term market growth.
Conclusion
The nasal antiseptics market is positioned for steady expansion, supported by rising infection prevention awareness, increasing surgical volumes, and growing demand for non-systemic antimicrobial solutions. Scientifically validated formulations, particularly iodine-based and spray formats, continue to drive clinical adoption across hospitals and community settings.
North America remains a key revenue contributor due to strong healthcare infrastructure and preventive care initiatives. Ongoing research into alcohol-based and antiviral applications is expected to broaden use cases beyond surgical decolonization. Overall, nasal antiseptics represent a practical, evidence-based approach to reducing microbial burden and strengthening global infection control strategies.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



