Table of Contents
Overview
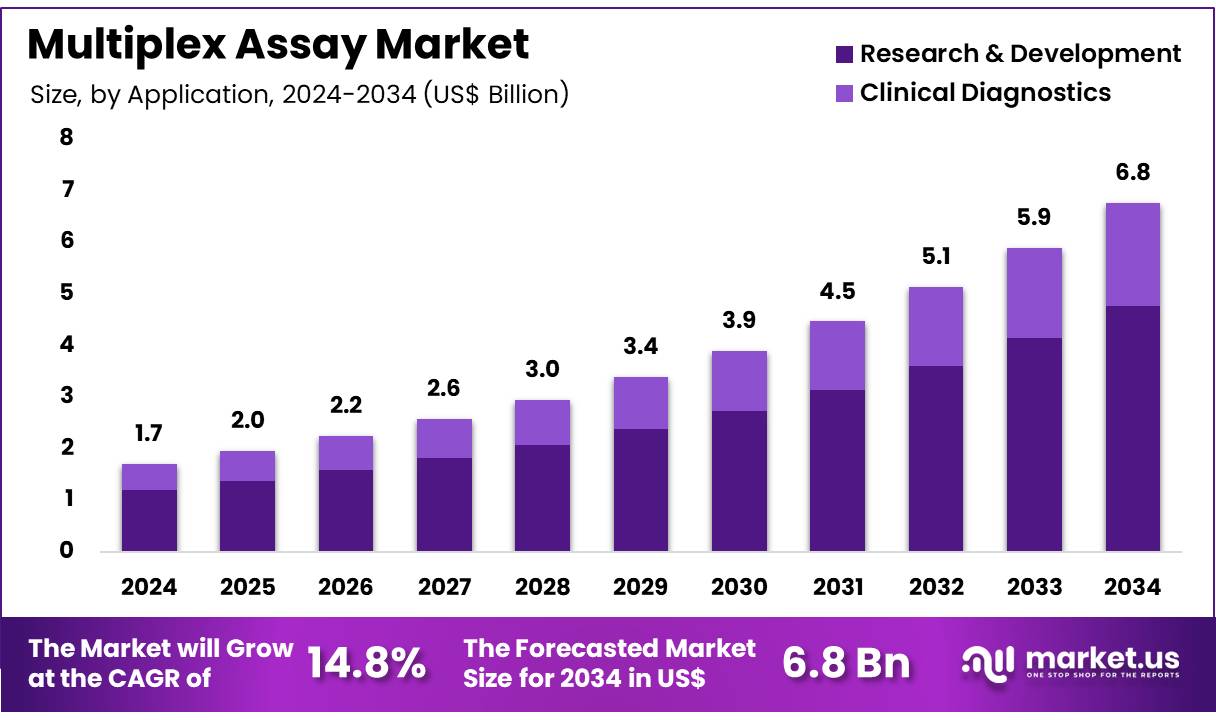
New York, NY – Dec 01, 2025 – Global Multiplex Assay Market size is expected to be worth around US$ 6.8 Billion by 2034 from US$ 1.7 Billion in 2024, growing at a CAGR of 14.8% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 38.6% share with a revenue of US$ 0.7 Billion.
The development of a multiplex assay has been undertaken to enable the simultaneous detection and quantification of multiple biomarkers within a single analytical run. This formation has been designed to improve laboratory efficiency, reduce sample consumption, and strengthen clinical and research decision-making. The integration of multiple analytes into one workflow has been recognized as a significant advancement in modern diagnostics.
The formation of the multiplex assay has been structured through the selection of target biomarkers, the optimization of reagent composition, and the establishment of standardized detection conditions. A high-sensitivity platform has been adopted to ensure that low-abundance biomarkers can be measured with precision. Cross-reactivity has been minimized through the careful validation of antibodies and probes, which has ensured reliable performance across diverse sample types.
The workflow has been developed to support applications in disease profiling, therapeutic monitoring, and translational research. The technology offers cost-efficient analysis, as the consolidation of assays into a single reaction reduces reagent use and turnaround time. The adoption of multiplex formats has also been associated with higher throughput, enabling laboratories to process larger sample volumes without compromising accuracy.
The launch of this multiplex assay is expected to support healthcare institutions, research organizations, and diagnostic developers in generating faster and more comprehensive insights. Its formation reflects continued innovation in biomarker analysis and demonstrates a commitment to advancing diagnostic capabilities. The assay is positioned to contribute to improved patient outcomes and greater scientific understanding across multiple disease areas.
Key Takeaways
- The multiplex assay market generated US$ 1.7 billion in 2024, and a CAGR of 14.8% has been observed. The market is projected to reach US$ 6.8 billion by 2033.
- The product type category includes consumables, software, and instruments, with consumables leading at 58.2% of the market share in 2024.
- In terms of technology, the market is classified into flow cytometry, multiplex real-time PCR, luminescence, fluorescence detection, and others. Flow cytometry accounted for 47.6% of the total share.
- The application segment comprises research & development and clinical diagnostics, with research & development dominating at 70.4% of the revenue share.
- The type segment includes protein multiplex assays, nucleic acid multiplex assays, and cell-based multiplex assays, where protein multiplex assays led with a 52.1% revenue contribution.
- Based on the end-user category, the market is divided into pharmaceutical & biotechnology companies, research & academic institutes, hospitals & diagnostic laboratories, and others. Pharmaceutical & biotechnology companies held 61.9% of the share.
- North America emerged as the leading regional market, capturing 38.6% of the global share in 2024.
Regional Analysis
North America is Leading the Multiplex Assay Market
North America accounted for the largest revenue share of 38.6%, supported by the expanding use of CRISPR technology in therapeutic development and academic research. Strong institutional funding has played a key role in driving regional growth. In fiscal year 2023, the National Institutes of Health (NIH) allocated US$ 34.9 billion to extramural research grants, a portion of which supported CRISPR-Cas9–based projects. This sustained investment reflects a strong commitment to advancing gene-based therapeutic innovation, thereby increasing the demand for multiplex assay technologies.
The region has also experienced a rise in clinical trials conducted by biotechnology companies focusing on CRISPR-driven treatments for cancer and genetic disorders. This trend has strengthened the need for advanced analytical platforms capable of supporting high-precision biomarker evaluation. Regulatory clarity provided by the U.S. Food and Drug Administration (FDA) regarding gene-edited therapy pathways has further encouraged research activity and capital inflow, contributing to market expansion across North America.
Asia Pacific is Expected to Register the Fastest CAGR
The Asia Pacific region is projected to record the highest CAGR during the forecast period, driven by rising investments in biotechnology across China, Japan, and South Korea. These countries have implemented strong government-backed initiatives promoting advancements in gene-editing technologies for both healthcare and agricultural applications. China’s commitment to scientific progress is illustrated by its R&D spending, which reached 2.54% of GDP in 2022, according to the National Bureau of Statistics of China.
Increasing cases of genetic disorders and expanding collaborations between regional biotech firms and global research institutions are expected to accelerate the adoption of CRISPR-related tools. This combination of strategic investment, supportive policies, and growing genomic research activity positions Asia Pacific for substantial growth in the multiplex assay market throughout the forecast period.
Emerging Trends
- Digital PCR–based multiplexing has gained momentum due to its ability to provide absolute quantification without calibration curves. By partitioning samples into numerous micro-reactions, it enables sensitive detection of low-abundance targets and improved resistance to inhibitors, particularly benefiting infectious disease diagnostics.
- Machine learning–enhanced assay design is being integrated with high-throughput multiplex platforms to optimize assay performance and interpret complex amplification patterns. Trained models improve detection limits, specificity, and analysis speed, supporting advanced identification of antimicrobial-resistance genes and critical biomarkers.
- Microfluidic lab-on-a-chip devices are being adopted to miniaturize multiplex immunoassays for point-of-care use. These capillary-driven systems utilize dry, antibody-coated beads to preserve analytes at collection, enabling centralized analysis with high sensitivity and minimal reagent consumption.
- Bead-based multiplex immunoassays continue to expand in serological and immunological applications due to their ability to measure multiple analytes simultaneously. Their high-throughput nature reduces sample volume needs and assay time while maintaining strong concordance with single-target methods.
- Expanded respiratory pathogen panels are increasingly used to detect SARS-CoV-2, influenza A/B, RSV, and additional respiratory agents in one test. These multi-target RT-PCR panels support efficient differential diagnosis and have demonstrated high concordance with individual FDA-approved assays.
Use Cases
- The CDC Influenza SARS-CoV-2 Multiplex Assay, authorized on July 2, 2020, supports simultaneous detection of influenza A, influenza B, and SARS-CoV-2. Its distribution to public health laboratories has streamlined workflows and strengthened surveillance capabilities.
- The HIV-1 Multiplex Incidence Assay uses bead-based technology to measure antibody levels and avidity to several HIV antigens, providing strong sensitivity and specificity for recent infections. Its application in outbreak investigations has supported timely epidemic monitoring.
- The CDC DENV-1-4 RT-PCR Multiplex Assay identifies and differentiates all four dengue virus serotypes from early-stage serum or plasma samples, generating valuable epidemiological insights for dengue-endemic regions and supporting early diagnostic confirmation.
- Multiplex PCR amplicon sequencing for tickborne pathogens enables simultaneous detection of key vector-associated organisms in environmental samples. Its adoption within national surveillance programs enhances monitoring of tickborne disease risks and informs targeted public health interventions.
Frequently Asked Questions on Multiplex Assay
- How does a multiplex assay work?
A multiplex assay functions by combining multiple analyte-specific reagents in a single reaction. Distinct detection signals are produced for each biomarker, allowing accurate measurement of several targets without separate tests, thereby improving overall process efficiency. - What are the main types of multiplex assays?
The primary types include protein multiplex assays, nucleic acid multiplex assays, and cell-based multiplex assays. These formats support diverse analytical needs, ranging from protein profiling to gene expression analysis and cell signaling studies across research and clinical applications. - What are the key applications of multiplex assays?
Multiplex assays are widely used in research and development, clinical diagnostics, drug discovery, immunology, oncology, and infectious disease studies. Their ability to evaluate multiple biomarkers simultaneously supports comprehensive biological insights and enhances data-driven decision-making. - Which segment dominates the multiplex assay market?
In 2024, the research and development segment dominated the market due to extensive use of multiplex technologies in biomarker discovery, therapeutic development programmes, and translational research activities, which require efficient multi-analyte testing capabilities. - Which product type leads the multiplex assay market?
Consumables hold the largest share of the product segment, as they are frequently required for repeated assay runs. Their recurrent demand in laboratories supports sustained revenue generation and positions the consumables category as the dominant contributor. - What technology is most widely used in multiplex assays?
Flow cytometry is the leading technology due to its high sensitivity, ability to analyse complex cell populations, and suitability for multi-parametric measurements. Its broad adoption in research and clinical workflows supports strong market preference. - Which region dominates the multiplex assay market?
North America leads the global market owing to strong research funding, advanced biotechnology infrastructure, and high adoption of innovative diagnostic technologies. The presence of major industry players further supports regional market strength. - Who are the primary end-users of multiplex assay technologies?
Pharmaceutical and biotechnology companies represent the major end-users, supported by extensive use of multiplex testing for drug discovery, development, and validation processes. Research institutes and diagnostic laboratories also contribute significantly to market adoption.
Conclusion
The multiplex assay market is positioned for sustained growth, supported by strong technological advancement, expanding research activities, and increasing clinical adoption. Rising demand for high-throughput biomarker analysis, combined with innovations such as digital PCR, machine learning integration, and microfluidic devices, continues to strengthen market momentum.
North America maintains a leading position due to significant research funding, while Asia Pacific is set for the fastest expansion driven by growing biotechnology investment. The market’s broad applications across diagnostics, therapeutic development, and surveillance underscore its strategic importance, positioning multiplex assays as essential tools for future scientific and clinical progress.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



