Table of Contents
Overview
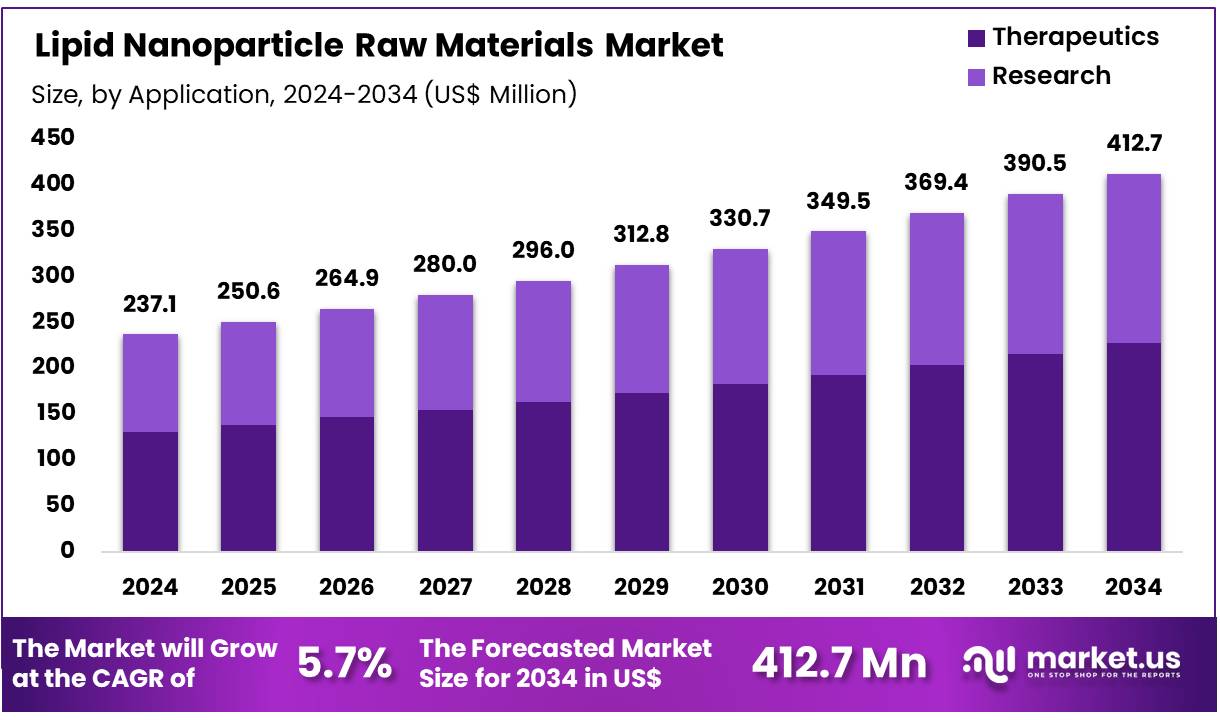
New York, NY – Dec 01, 2025 – Global Lipid Nanoparticle Raw Materials Market size is expected to be worth around US$ 412.7 Million by 2034 from US$ 237.1 Million in 2024, growing at a CAGR of 5.7% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 40.4% share with a revenue of US$ 95.8 Million.
The global demand for lipid nanoparticle (LNP) raw materials has been expanding as their application in mRNA therapeutics, vaccines, and gene-delivery systems continues to accelerate. The formation of LNP systems is supported by four primary raw material classes: ionizable lipids, phospholipids, cholesterol, and polyethylene glycol (PEG)-lipids. Each component plays a critical role in ensuring stability, bioavailability, and delivery efficiency.
Ionizable lipids are used for effective nucleic acid encapsulation, and their adoption has been increasing as mRNA-based drug development progresses. Phospholipids contribute structural integrity, while cholesterol enhances membrane fluidity and improves particle stability. PEG-lipids support extended circulation time, thereby improving pharmacokinetic performance. The growth of the market has been driven by rising R&D investment and robust interest from biopharmaceutical manufacturers.
The market has been strengthened by advancements in formulation technologies, which have enabled improved particle uniformity and higher encapsulation efficiency. Increasing interest in precision medicine has encouraged significant innovation in lipid design and synthesis. The supply chain for these raw materials has been experiencing strategic expansion due to higher production requirements for mRNA vaccines and emerging nucleic acid therapies.
Regulatory focus on quality, purity, and consistency has further shaped the competitive landscape, with leading suppliers investing in GMP-compliant manufacturing capabilities. Continuous innovation in lipid chemistry is expected to support broader therapeutic applications, thereby reinforcing the long-term growth outlook for LNP raw material suppliers worldwide.
Key Takeaways
- In 2024, the lipid nanoparticle raw materials market recorded revenue of US$ 237.1 million, growing at a CAGR of 5.7%, and is projected to reach US$ 412.7 billion by 2033.
- By product type, the market is categorized into ionizable lipids, kits, reagents, and others, with kits dominating the segment in 2024 with a 48.7% share.
- Based on disease indication, the market is segmented into cancer, infectious diseases, blood diseases, and others, where infectious diseases accounted for a 42.6% share.
- In terms of application, the market is divided into therapeutics and research, with the therapeutics segment leading at 55.3% of total revenue.
- The end-user segmentation includes pharmaceutical & biotechnology companies, academic & research institutes, and others, with pharmaceutical & biotechnology companies representing the largest share at 60.4%.
- North America held the leading regional position in 2024 with a 40.4% market share.
Market Segmentation
- Product Type Analysis: The kits segment accounted for 48.7% of the total market share in 2023. This position was supported by the rising requirement for standardized formulations in lipid nanoparticle (LNP) manufacturing. Kits have been widely adopted due to their ability to simplify production workflows and enhance batch-to-batch consistency in drug delivery systems. The increasing utilization of RNA-based therapies, particularly mRNA vaccines, further strengthened this segment. Continuous advancements in kit formulation technologies, aimed at improving nanoparticle stability and delivery performance, are expected to support broader adoption across pharmaceutical companies and research institutions worldwide.
- Disease Indication Analysis: The infectious diseases segment represented 42.6% of the market in 2023. This prominence was driven by the expanding use of LNPs in RNA-based therapies designed to target viral infections. The success demonstrated by mRNA vaccines during the COVID-19 pandemic accelerated the integration of LNP platforms into infectious disease management strategies. Their adaptability in delivering nucleic acids and therapeutic agents is expected to support expanded clinical application for existing and emerging infectious disease challenges on a global scale.
- Application Analysis: The therapeutics segment held the leading position with a 55.3% revenue share in 2023. Lipid nanoparticles have become integral to the development of RNA-based therapeutics, including mRNA vaccines and gene therapy products. Their high delivery efficiency and ability to transport diverse active molecules make them critical for addressing complex disease conditions. Ongoing research activities, supported by favorable clinical trial outcomes, are anticipated to reinforce the use of LNPs in therapeutic applications, particularly in the fields of oncology and rare genetic disorders over the forecast period.
- End-user Analysis: Pharmaceutical and biotechnology companies accounted for 60.4% of the end-user share in 2023, driven by increasing investment in RNA-based drug development pipelines. Lipid nanoparticles have become a foundational component of advanced drug delivery platforms, especially for vaccines and gene therapy solutions. The achievements observed with COVID-19 mRNA vaccines further validated the commercial and clinical potential of LNP technologies. Continued research initiatives and commercialization activities are projected to elevate demand for LNP-related raw materials within this end-user segment, supporting sustained market growth.
Regional Analysis
North America Leading the Lipid Nanoparticle Raw Materials Market
North America maintained the largest share of the lipid nanoparticle raw materials market, accounting for 40.4% of total revenue. The region’s leadership can be attributed to the ongoing advancement of mRNA-based therapies. Multiple mRNA products have received FDA approval in recent years, and each relies on lipid nanoparticle delivery systems, thereby increasing the demand for high-quality specialized lipids. The regulatory support provided through these approvals has encouraged further innovation and investment across the LNP ecosystem.
In addition, significant research funding from the National Institutes of Health (NIH) has reinforced market growth. Allocations reported in the NIH Data Book for fiscal year 2023 highlight substantial investments in projects aimed at improving drug delivery technologies, including LNP platforms. This funding supports the development of new formulation approaches and the exploration of advanced raw material options, strengthening the region’s position in the global market.
Asia Pacific Expected to Register the Highest CAGR
The Asia Pacific region is projected to record the fastest growth rate during the forecast period. Increasing research activity in mRNA therapeutics, combined with expanding government support for biotechnology development in countries such as China and Japan, is contributing to rising demand for LNP raw materials. National initiatives promoting biopharmaceutical innovation are expected to accelerate the adoption of advanced delivery technologies such as lipid nanoparticles.
Moreover, growing collaboration between global pharmaceutical companies and regional research institutions in mRNA vaccine and therapeutic development is anticipated to further enhance market demand. The expanding focus on personalized medicine across the region is also expected to increase reliance on efficient delivery platforms, thereby supporting strong market expansion in Asia Pacific.
Emerging Trend
- Regulatory Standardization of LNP Quality: Regulatory authorities have intensified efforts to establish unified standards for lipid nanoparticle (LNP) materials. In October 2022, the U.S. FDA conducted a public workshop to outline quality, safety, and efficacy criteria for prophylactic LNP–mRNA vaccines. This initiative was driven by the increasing need for harmonized manufacturing practices across developers to ensure consistent performance and safety outcomes.
- Platform Technology Designation for LNPs: LNP platforms used in mRNA vaccine and gene therapy applications have been recognized under the FDA’s Platform Technology Designation Program. This designation enables accelerated review of updates related to LNP composition, manufacturing methods, and control strategies. The inclusion of LNPs within this framework reflects growing regulatory confidence in their use as reusable, adaptable delivery systems.
- Diversification of Lipid Chemistries: The market is experiencing a shift toward the development of novel lipid chemistries. Optimization of techniques such as ethanol-loading and T-tube mixing has facilitated the creation of ionizable and cationic lipids with enhanced payload encapsulation and improved endosomal escape. These refinements have supported higher loading efficiencies, particularly for nanoparticle sizes below 100 nm.
- Expansion Beyond Vaccines: The application of LNP raw materials has expanded into therapeutic areas beyond vaccines. Increasing utilization in siRNA delivery and oncology therapeutics has been observed, supported by recent studies demonstrating the effectiveness of LNP-based RNA therapies in breast cancer. This progression highlights the versatility of LNPs in transporting diverse nucleic acid molecules for various clinical indications.
- Adoption of Continuous Manufacturing: Continuous and scalable manufacturing systems are being adopted to address growing global demand for LNP-enabled products. The FDA has evaluated continuous-flow production methods for mRNA–LNP COVID-19 vaccines to enhance efficiency and reduce batch-to-batch variability. These advancements are expected to support more robust and cost-effective large-scale manufacturing processes.
Frequently Asked Questions on Lipid Nanoparticle Raw Materials
- Why are ionizable lipids important in LNP formulation?
Ionizable lipids are considered vital because they enable efficient RNA complexation at acidic pH and facilitate endosomal escape after cell uptake. Their tunable charge characteristics have supported strong delivery efficiency across multiple nucleic acid-based therapeutic development programs. - What role does cholesterol play in LNP structure?
Cholesterol is used to enhance membrane fluidity and structural integrity in the nanoparticle system. Its inclusion helps stabilize particle morphology, supports efficient payload protection, and improves overall delivery performance for mRNA and siRNA pharmaceutical applications. - Why is PEG-lipid included in LNP systems?
PEG-lipids provide steric stabilization, reduce aggregation, and support predictable particle size distribution. Controlled PEG concentration allows optimized circulation time and enhances pharmacokinetics, enabling more reliable biodistribution profiles for various nucleic acid-based therapeutic modalities. - What quality requirements apply to LNP raw materials?
LNP raw materials generally require high purity, controlled particle characteristics, and strict adherence to GMP manufacturing standards. Regulatory expectations emphasize consistency, traceability, and low impurity levels to ensure safe, reproducible performance in clinical and commercial pharmaceutical products. - What factors are driving growth in the LNP raw materials market?
Market growth is driven by rising demand for mRNA vaccines, increasing adoption of nucleic acid therapeutics, and expanding R&D investments. Rapid progress in gene therapy pipelines has also resulted in greater consumption of high-grade lipid excipients worldwide. - Which applications are contributing most to market expansion?
Strong expansion has been observed in mRNA vaccines, genetic medicines, personalized oncology treatments, and emerging protein-replacement therapies. These applications rely heavily on LNP systems, increasing global demand for specialized ionizable lipids and high-purity phospholipid components. - Which regions are leading the LNP raw materials market?
North America and Europe currently lead due to advanced biopharmaceutical infrastructure, large R&D expenditures, and strong regulatory support. Asia-Pacific is experiencing accelerated growth driven by vaccine manufacturing capacity expansion and increasing regional investments in RNA therapeutics.
Conclusion
The global lipid nanoparticle raw materials market has been expanding steadily as demand for mRNA vaccines, gene therapies, and nucleic acid–based therapeutics increases. Growth has been supported by rising R&D investments, advancement in formulation technologies, and strengthened regulatory focus on quality and GMP production.
Kits, infectious disease applications, and pharmaceutical companies remain dominant segments, while North America leads regionally. The market outlook is positive, driven by innovation in lipid chemistries, broader therapeutic adoption beyond vaccines, and the shift toward continuous manufacturing. These developments are expected to sustain long-term demand for high-purity LNP raw materials worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



