Table of Contents
Overview
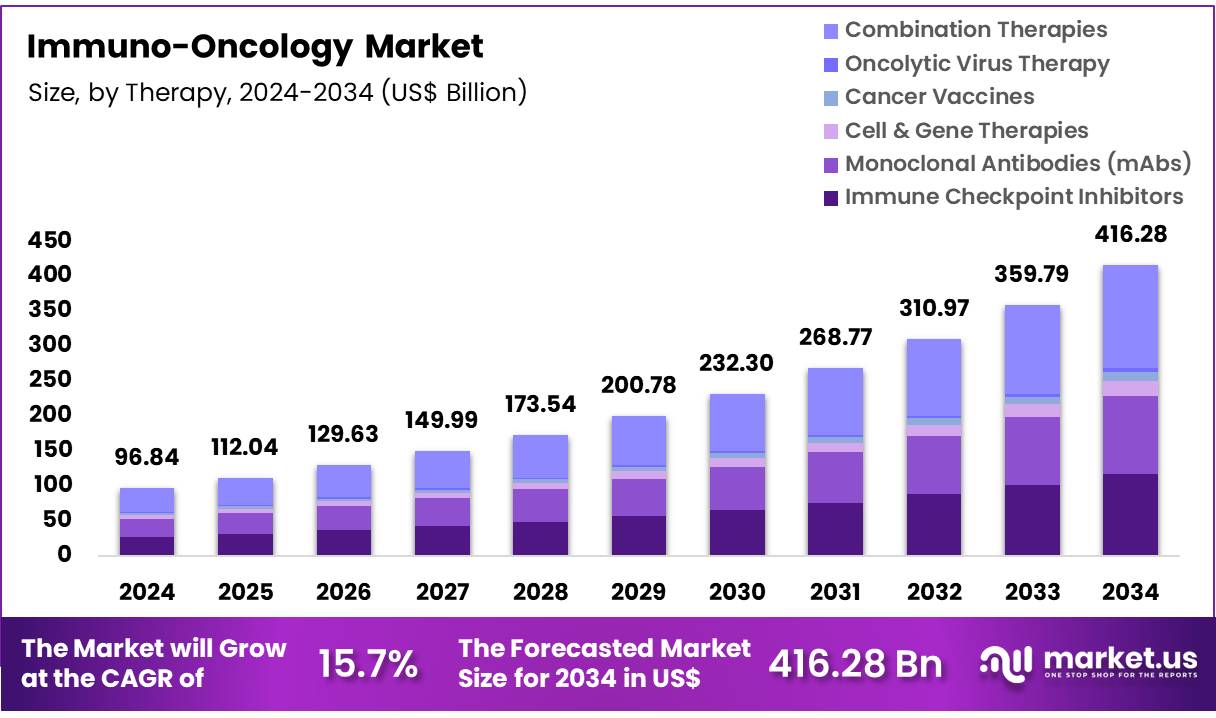
New York, NY – Nov 12, 2025 – Global Immuno-Oncology Market size is expected to be worth around US$ 96.84 Billion by 2034 from US$ 416.28 Billion in 2024, growing at a CAGR of 15.7% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 47.8% share with a revenue of US$ 46.29 Billion.
Immuno-oncology represents a transformative advancement in cancer therapy by harnessing the body’s immune system to identify and eliminate malignant cells. Unlike traditional treatments such as chemotherapy and radiation, which directly target cancer cells, immuno-oncology enhances the immune response to achieve durable and targeted tumor control. Key therapeutic approaches include immune checkpoint inhibitors, cancer vaccines, and adoptive cell transfer therapies, which have demonstrated significant improvements in survival rates across various cancer types.
The growth of the immuno-oncology market is being driven by an increasing prevalence of cancer, rising demand for personalized medicine, and continuous innovation in biologics and monoclonal antibodies. Advancements in biomarkers and companion diagnostics are further enabling precise patient selection, thereby improving treatment efficacy and safety profiles. Leading pharmaceutical companies and research institutions are intensifying collaborations to accelerate clinical trials and expand therapeutic applications beyond melanoma and lung cancer.
Ongoing research is also exploring the combination of immuno-oncology with conventional therapies to enhance response rates and overcome resistance. With a growing number of regulatory approvals and expanding reimbursement coverage, the global immuno-oncology landscape is poised for sustained growth. This innovative approach continues to redefine cancer care, offering renewed hope for patients and setting new benchmarks for therapeutic outcomes.
Key Takeaways
- The global immuno-oncology market was valued at USD 96.84 billion in 2024 and is projected to achieve a value of USD 416.28 billion by 2034, registering a compound annual growth rate (CAGR) of 15.7% during the forecast period.
- By therapy, the market is segmented into immune checkpoint inhibitors, monoclonal antibodies (mAbs), cell and gene therapies, cancer vaccines, oncolytic virus therapy, and combination therapies. Among these, combination therapies held the largest share in 2024, accounting for 35.4% of the global revenue.
- By indication, the market is divided into solid tumors and hematologic malignancies, with solid tumors dominating the segment by contributing 61.2% of the total market share in 2024.
- By stage or line of therapy, the market is categorized into first-line (frontline), second-line, third-line or later lines, adjuvant (post-surgery), and neoadjuvant (pre-surgery) therapies. The first-line therapy segment led the market with a 41.2% share in 2024.
- By distribution channel, the market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies accounted for the largest portion, representing 52.9% of the total share in 2024.
- Regionally, North America dominated the global immuno-oncology market, capturing 47.8% of total revenue in 2024, supported by advanced healthcare infrastructure, strong R&D investments, and high adoption of innovative cancer therapies.
Regional Analysis
North America continues to dominate the global immuno-oncology market, holding a 47.8% share in 2024. The rising prevalence of cancer, particularly colorectal, endometrial, breast, and other malignancies, has driven the growing demand for targeted and personalized therapies across the region. Favorable regulatory support from authorities such as the U.S. Food and Drug Administration (FDA) has further accelerated market expansion, with multiple approvals granted for advanced therapies, including immune checkpoint inhibitors like pembrolizumab and nivolumab.
According to estimates from the American Cancer Society (ACS), approximately 1.9 million new cancer cases are expected to be diagnosed in the United States in 2024, with nearly 609,000 deaths projected to occur due to cancer-related causes. Among these, breast cancer, lung cancer, prostate cancer, and colorectal cancer remain the most commonly diagnosed forms. Notably, breast cancer alone is anticipated to represent about 20% of all newly reported cancer cases in the country.
This growing disease burden, combined with strong research infrastructure, rising healthcare expenditure, and early adoption of immunotherapy-based treatment options, continues to reinforce North America’s leadership position in the global immuno-oncology landscape.
Emerging Trends in Immuno-Oncology
- Biomarker-Driven Drug Approvals: An increasing proportion of new cancer therapies are being approved based on molecular biomarkers rather than tumor site. Between January and March 2021, approximately 33% of the U.S. FDA’s novel drug approvals were linked to biomarkers such as PD-L1 expression or microsatellite instability (MSI) status, highlighting a major shift toward precision oncology.
- Expansion of TIL Therapy to Common Solid Tumors: Tumor-infiltrating lymphocyte (TIL) therapy, initially effective in melanoma, is now demonstrating efficacy in broader tumor types. In a phase 2 trial for metastatic gastrointestinal cancers, 23.5% of patients receiving selected TILs combined with pembrolizumab achieved tumor reduction, compared to 7.7% among those treated without pembrolizumab.
- Combination Checkpoint Inhibitor Regimens by Genetic Profile: Checkpoint inhibitor combinations are increasingly being tailored to genetic subtypes of cancer. On April 8, 2025, the FDA approved nivolumab in combination with ipilimumab for patients aged 12 years and older with unresectable or metastatic MSI-high colorectal cancer, marking a step toward genotype-guided immunotherapy.
- Advancements in Personalized Cell-Based Immunotherapies: Autologous, tumor-derived T cell therapies customized to each patient’s unique tumor characteristics are progressing rapidly. In February 2024, lifileucel (Amtagvi) became the first FDA-approved tumor-infiltrating lymphocyte–based therapy for metastatic melanoma, representing a milestone in individualized cancer treatment.
- Integration of Artificial Intelligence in Predictive Oncology: Artificial intelligence tools are increasingly being utilized to predict immunotherapy outcomes. The SCORPIO AI model, for instance, has demonstrated superior accuracy in identifying patients likely to respond to checkpoint inhibitors and in estimating overall survival, thereby supporting data-driven clinical decisions.
Use Cases in Immuno-Oncology
- Lifileucel for Metastatic Melanoma: In a global clinical study involving 73 patients, lifileucel achieved an objective response rate of 31.5%, comprising 4.1% complete and 27.4% partial responses. Among responders, 56.5% remained progression-free at six months, confirming the therapy’s durable efficacy.
- Dostarlimab for dMMR Rectal Cancer: A phase 2 clinical trial investigated dostarlimab monotherapy in 12 patients with mismatch repair–deficient (dMMR), locally advanced rectal adenocarcinoma. All patients (100%) achieved a complete clinical response without the need for chemoradiation or surgery after a median follow-up of 12 months.
- Selected TIL Therapy with Pembrolizumab in Gastrointestinal Tumors: In a cohort of 34 heavily pretreated patients with metastatic gastrointestinal cancers, 23.5% achieved tumor shrinkage of ≥30% when pembrolizumab was administered prior to selected TIL infusion. In comparison, only 7.7% of 39 patients responded to TIL therapy alone, underscoring the synergistic benefit of combination regimens.
- Nivolumab and Ipilimumab for MSI-High Colorectal Cancer: The combination of nivolumab and ipilimumab received FDA approval on April 8, 2025, for patients aged 12 and above with unresectable or metastatic MSI-high or dMMR colorectal cancer. MSI-high tumors represent approximately 2–3% of all metastatic colorectal cancer cases, making this approval a key development in targeted immunotherapy.
Frequently Asked Questions on Immuno-Oncology
- How does Immuno-Oncology differ from traditional cancer therapy?
Unlike chemotherapy or radiation that directly target tumors, Immuno-Oncology treatments stimulate the immune system to identify and destroy cancer cells, providing longer-lasting responses and fewer side effects in some patients. - What are the key types of Immuno-Oncology therapies?
Major therapy types include immune checkpoint inhibitors, monoclonal antibodies, cancer vaccines, and adoptive cell therapies such as CAR-T. These approaches enhance immune system activity against specific tumor antigens. - Which cancers are treated with Immuno-Oncology therapies?
Immuno-Oncology therapies have shown effectiveness in treating melanoma, lung cancer, lymphoma, and bladder cancer. Ongoing clinical trials are expanding applications to breast, colorectal, and prostate cancers. - What are immune checkpoint inhibitors?
Immune checkpoint inhibitors block proteins like PD-1, PD-L1, and CTLA-4 that suppress immune responses. By inhibiting these checkpoints, the immune system can attack cancer cells more effectively. - What is CAR-T cell therapy?
CAR-T therapy involves engineering a patient’s T-cells to express receptors that specifically target cancer cells. It has shown remarkable success in certain blood cancers like leukemia and lymphoma. - How are biomarkers used in Immuno-Oncology?
Biomarkers help identify patients likely to respond to immunotherapies. Common biomarkers include PD-L1 expression, tumor mutational burden, and microsatellite instability status. - Which regions dominate the Immuno-Oncology market?
North America holds the largest market share due to advanced healthcare infrastructure, strong R&D investments, and early adoption of innovative immunotherapies, followed by Europe and Asia-Pacific. - Who are the leading players in the Immuno-Oncology market?
Major players include Bristol-Myers Squibb, Merck & Co., Roche, Novartis, and AstraZeneca. These companies lead in checkpoint inhibitor development and novel combination therapies.
Conclusion
The global immuno-oncology market is witnessing robust expansion, driven by advances in immune-based therapies and personalized medicine. The rising prevalence of cancer, strong regulatory support, and increasing adoption of combination therapies are key contributors to growth. Breakthroughs in biomarkers, AI-driven predictive tools, and novel cell-based treatments are enhancing therapeutic precision and outcomes.
North America remains the dominant region due to high R&D investments and early adoption of innovative therapies. With a projected CAGR of 15.7% from 2024 to 2034, immuno-oncology is expected to redefine cancer treatment paradigms and establish long-term clinical and economic value worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



