Table of Contents
Overview
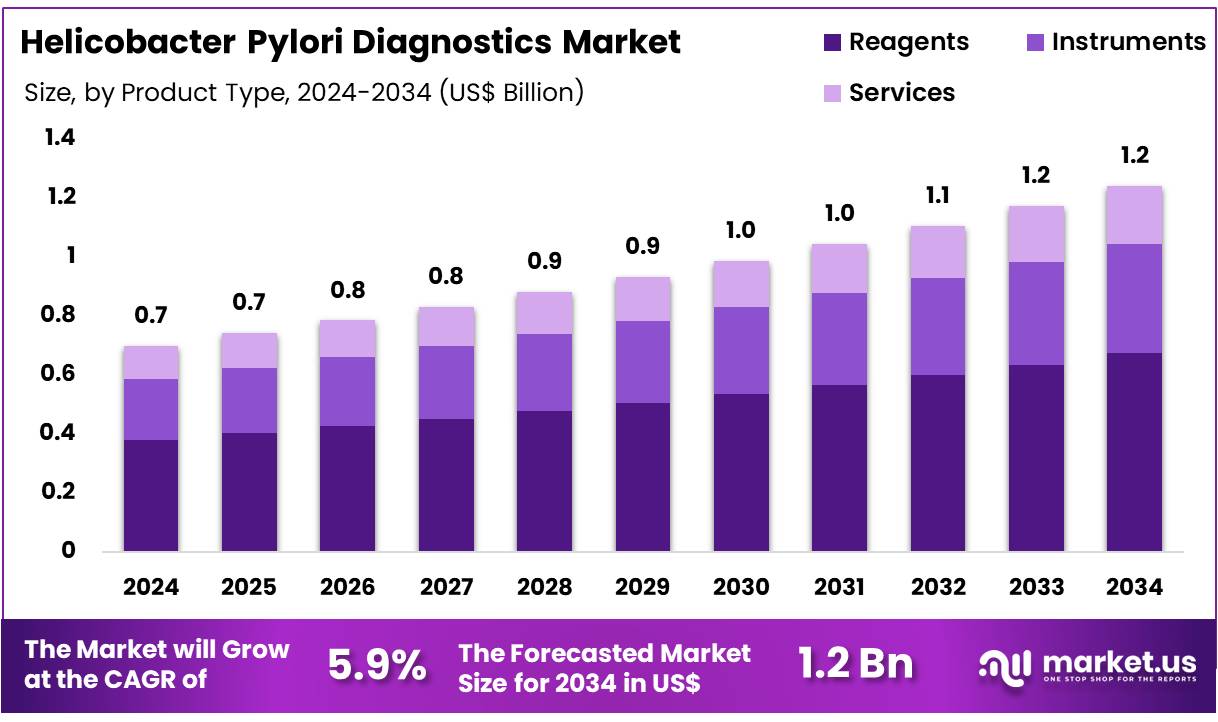
New York, NY – Dec 19, 2025 – Global Helicobacter Pylori Diagnostics Market size is expected to be worth around US$ 1.2 Billion by 2034 from US$ 0.7 Billion in 2024, growing at a CAGR of 5.9% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 46.7% share with a revenue of US$ 0.3 Billion.
The Helicobacter pylori (H. pylori) diagnostics segment plays a critical role in the detection and management of gastrointestinal disorders, particularly gastritis, peptic ulcer disease, and gastric cancer. H. pylori is a gram-negative bacterium that infects the stomach lining and is recognized as one of the most common chronic bacterial infections globally. Early and accurate diagnosis is considered essential for effective treatment and long-term disease prevention.
H. pylori diagnostics are broadly classified into non-invasive and invasive testing methods. Non-invasive diagnostics include urea breath tests, stool antigen tests, and serological assays, which are widely adopted due to their convenience, patient comfort, and suitability for large-scale screening. Among these, the urea breath test is regarded as a gold standard because of its high sensitivity and specificity. Stool antigen tests are increasingly utilized for both initial diagnosis and post-treatment monitoring.
Invasive diagnostic methods involve endoscopic procedures such as biopsy-based rapid urease tests, histology, and culture techniques. These methods are primarily used in clinical settings where endoscopy is already indicated and provide additional information on mucosal pathology and antibiotic susceptibility.
Technological advancements in diagnostic platforms, including molecular diagnostics and rapid test kits, are supporting improved accuracy and reduced turnaround times. The growth of the H. pylori diagnostics market is driven by rising prevalence of gastrointestinal diseases, increasing awareness of early diagnosis, and expanding access to healthcare services. Supportive clinical guidelines and growing demand for point-of-care testing are further strengthening adoption across hospitals, diagnostic laboratories, and specialty clinics.
Overall, H. pylori diagnostics continue to represent a vital component of preventive and therapeutic gastrointestinal healthcare.
Key Takeaways
- In 2024, the Helicobacter pylori diagnostics market generated revenue of US$ 0.7 billion and is projected to expand at a CAGR of 5.9%, reaching an estimated value of US$ 1.2 billion by 2034.
- By product type, the market is segmented into instruments, services, and reagents, with the reagents segment leading in 2024 by accounting for 54.2% of total market revenue.
- Based on application, the market is categorized into immunoassays, molecular diagnostics, and point-of-care testing. Among these, immunoassays emerged as the leading application, capturing a market share of 45.1%.
- In terms of end users, the market is divided into hospitals, diagnostic laboratories, and clinics. Hospitals dominated the segment, contributing the highest revenue share of 58.9% to the overall market.
- Regionally, North America maintained its leadership position in 2024, representing 46.7% of the global Helicobacter pylori diagnostics market.
Regional Analysis
North America continues to dominate the Helicobacter pylori diagnostics market, accounting for approximately 46.7% of the global market share in 2024. This leadership position is supported by the sustained prevalence of H. pylori infections, rising clinical awareness of its link with gastrointestinal disorders, and continuous advancements in diagnostic technologies.
A long-term meta-analysis covering 1980–2022 estimated the regional prevalence at 36.2%, while data from the Veterans Health Administration indicated that 25.8% of tested individuals were positive between 1999 and 2018. The strong focus on early diagnosis and eradication to reduce the risk of peptic ulcers and gastric cancer has further strengthened diagnostic demand.
In addition, stomach cancer remains a major concern in the US, with nearly 11,000 deaths reported in 2022. Major players such as Abbott have benefited from this demand, with its Diagnostics segment generating US$3.83 billion in US sales in 2024.
In contrast, the Asia Pacific region is projected to register the highest compound annual growth rate during the forecast period. This growth is driven by high infection prevalence, a large gastric cancer burden, and improving healthcare access. A recent meta-analysis reported a 42.8% prevalence rate in mainland China, while China alone recorded around 358,700 new stomach cancer cases in 2022. Strengthening public health initiatives and strong participation from global diagnostic companies are expected to support continued market expansion across the region.
Emerging Trends
- Shift Toward Non-Invasive Diagnostic Methods: A notable transition toward non-invasive diagnostic techniques for Helicobacter pylori detection is being observed. Diagnostic tools such as the urea breath test and stool antigen test are increasingly preferred due to their high diagnostic accuracy and improved patient compliance. These methods reduce dependence on invasive procedures such as endoscopy, thereby enhancing accessibility and minimizing patient discomfort.
- Expansion of Point-of-Care Diagnostic Solutions: The adoption of rapid point-of-care diagnostic tests is gaining momentum. These solutions, commonly based on immunochromatographic platforms, enable on-site detection of H. pylori without the requirement for advanced laboratory infrastructure. Rapid turnaround times support timely clinical decision-making, particularly in primary care and resource-constrained healthcare settings.
- Advancements in Molecular Diagnostic Technologies: Molecular diagnostics, particularly polymerase chain reaction (PCR)-based methods, are increasingly being utilized for H. pylori detection. These technologies offer superior sensitivity and specificity by identifying bacterial genetic material. In addition, molecular assays enable the detection of antibiotic resistance markers, supporting personalized treatment strategies and improving eradication outcomes.
- Integration of Artificial Intelligence in Diagnostics: Artificial intelligence is emerging as a supportive tool in H. pylori diagnostics. AI-driven algorithms are being applied to analyze endoscopic images, histopathological data, and patient clinical profiles. This integration has the potential to improve diagnostic precision, enable earlier detection of infection-related abnormalities, and reduce the diagnostic burden on healthcare professionals.
- Evolution of Serological Testing Approaches: While conventional serological tests focus on antibody detection, newer methodologies emphasize the identification of specific antigens and virulence-associated factors. This evolution aims to enhance diagnostic accuracy and provide deeper insights into infection activity and strain pathogenicity, supporting more informed clinical decision-making.
Use Cases
- Routine Screening in High-Prevalence Regions: In geographic regions with elevated H. pylori prevalence, routine population screening programs are widely implemented. Non-invasive diagnostic methods, including urea breath tests and stool antigen tests, are commonly employed. In several Asian countries with high gastric cancer incidence, early detection through systematic screening contributes to reduced disease burden and improved long-term outcomes.
- Evaluation of Dyspeptic Symptoms: Patients presenting with chronic dyspeptic symptoms, such as upper abdominal pain, bloating, or nausea, are frequently evaluated for H. pylori infection. Accurate identification using diagnostic tools such as rapid urease tests or non-invasive assays supports targeted antibiotic therapy, which is associated with symptom improvement in a significant proportion of cases.
- Post-Therapy Eradication Assessment: Confirmation of bacterial eradication following treatment is a critical component of H. pylori management. Non-invasive diagnostic tests, particularly the urea breath test and stool antigen test, are routinely conducted four to six weeks after therapy completion to verify treatment success and reduce the risk of disease recurrence or progression.
- Pre-Endoscopy Clinical Assessment: H. pylori testing is often conducted prior to upper gastrointestinal endoscopy, especially in patients presenting with alarm symptoms such as unexplained weight loss or gastrointestinal bleeding. Detection of active infection can influence endoscopic interpretation and guide further diagnostic or therapeutic interventions, including targeted biopsies.
- Public Health Surveillance and Epidemiological Research: Diagnostic testing for H. pylori plays a vital role in epidemiological studies and public health monitoring programs. Large-scale data collection enables assessment of infection prevalence, identification of population-level risk factors, and formulation of evidence-based public health strategies aimed at reducing infection rates and associated complications.
Frequently Asked Questions on Helicobacter Pylori Diagnostics
- What are the commonly used diagnostic tests for Helicobacter pylori?
Common diagnostic methods include urea breath tests, stool antigen tests, serology tests, endoscopic biopsy, and rapid urease tests. Non-invasive tests are widely preferred due to patient comfort, accuracy, and suitability for large-scale screening programs. - How accurate are non-invasive Helicobacter pylori diagnostic tests?
Non-invasive tests such as urea breath tests and stool antigen tests demonstrate high sensitivity and specificity, often exceeding 90%. Accuracy may vary based on patient preparation, recent antibiotic use, and proton pump inhibitor consumption. - When is invasive testing for Helicobacter pylori recommended?
Invasive diagnostic methods are recommended when patients present with alarm symptoms, gastrointestinal bleeding, or suspected malignancy. Endoscopic biopsy allows direct visualization and histological confirmation, supporting comprehensive clinical evaluation and treatment planning. - Can Helicobacter pylori infection recur after treatment?
Recurrence of Helicobacter pylori infection can occur due to reinfection or incomplete eradication. Follow-up testing is advised after therapy completion to confirm successful eradication and reduce the risk of recurrent gastric disorders. - What factors are driving the growth of the Helicobacter pylori diagnostics market?
Market growth is driven by rising prevalence of gastrointestinal disorders, increasing awareness of early diagnosis, technological advancements in non-invasive testing, and expanding healthcare infrastructure in emerging economies worldwide. - Which diagnostic segment holds the largest market share?
Non-invasive diagnostic tests dominate the market due to their high accuracy, cost-effectiveness, and patient convenience. Urea breath tests and stool antigen tests are particularly favored in both clinical and screening settings. - How is technological innovation influencing the market?
Technological innovation has led to improved test sensitivity, faster turnaround times, and point-of-care solutions. These advancements are enhancing diagnostic efficiency and supporting broader adoption across hospitals, diagnostic laboratories, and outpatient clinics. - What regional trends are observed in the Helicobacter pylori diagnostics market?
North America and Europe hold significant market shares due to advanced healthcare systems, while Asia-Pacific is experiencing rapid growth driven by high infection prevalence, expanding diagnostic access, and increased healthcare expenditure.
Conclusion
The Helicobacter pylori diagnostics market remains a critical component of gastrointestinal disease management, supported by the high global burden of infection and its strong association with peptic ulcers and gastric cancer. Market growth is being driven by increasing reliance on non-invasive diagnostic methods, expanding point-of-care testing, and continued advancements in molecular technologies.
Strong clinical awareness and supportive guidelines are reinforcing routine screening and post-treatment monitoring. While North America continues to lead in market share, rapid growth in Asia Pacific reflects unmet diagnostic needs and rising healthcare access. Overall, sustained innovation and early-detection strategies are expected to support steady market expansion.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



